Chinese Journal of Tissue Engineering Research ›› 2021, Vol. 25 ›› Issue (2): 258-263.doi: 10.3969/j.issn.2095-4344.2976
Previous Articles Next Articles
Chemerin, a pro-inflammatory adipokine, regulates chondrocyte proliferation and metabolism by increasing production of nitric oxide
Yu Chengshuai, Du Gang, Pang Shenning, Lao Shan
- First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China
-
Received:2019-12-19Revised:2019-12-24Accepted:2020-02-12Online:2021-01-18Published:2020-11-21 -
Contact:Lao Shan, MD, Chief physician, First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China -
About author:Yu Chengshuai, Master candidate, First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China -
Supported by:the National Natural Science Foundation of China, No. 8156090038; the Natural Science Foundation of Guangxi Zhuang Autonomous Region, No. 2017GXNSFAA198159; the Scientific Research Project of Guangxi Zhuang Autonomous Region, No. AB19110030
CLC Number:
Cite this article
Yu Chengshuai, Du Gang, Pang Shenning, Lao Shan. Chemerin, a pro-inflammatory adipokine, regulates chondrocyte proliferation and metabolism by increasing production of nitric oxide[J]. Chinese Journal of Tissue Engineering Research, 2021, 25(2): 258-263.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

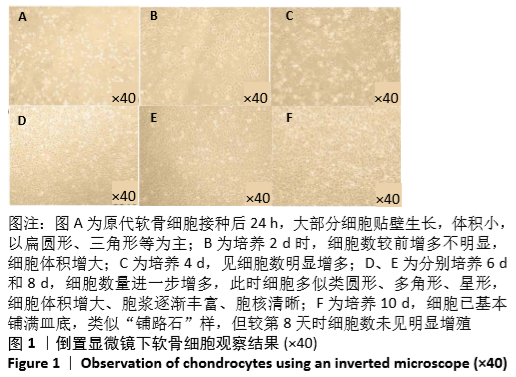
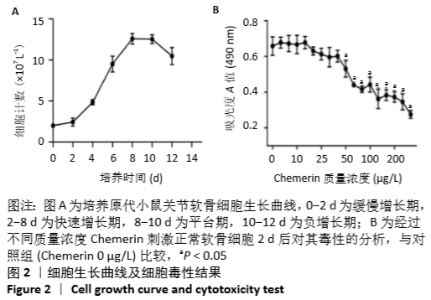
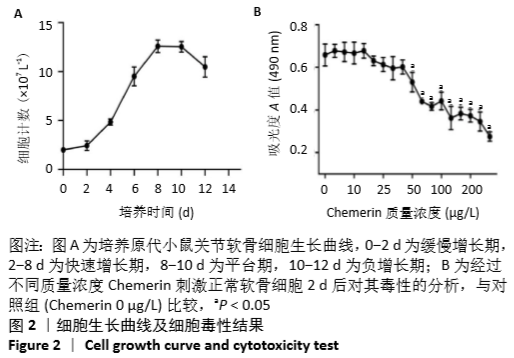
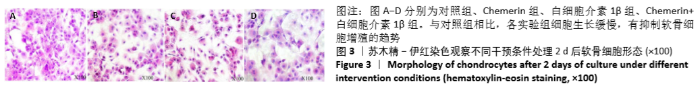
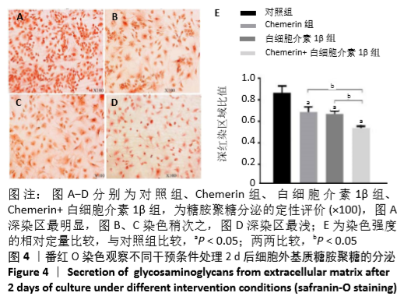
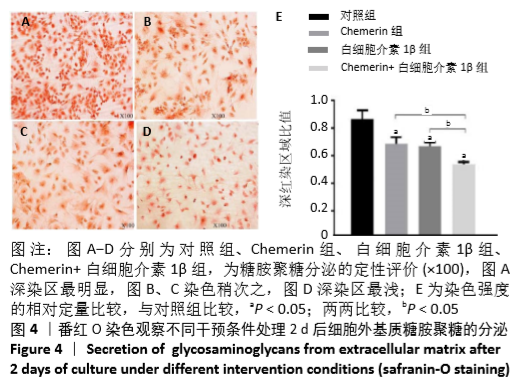
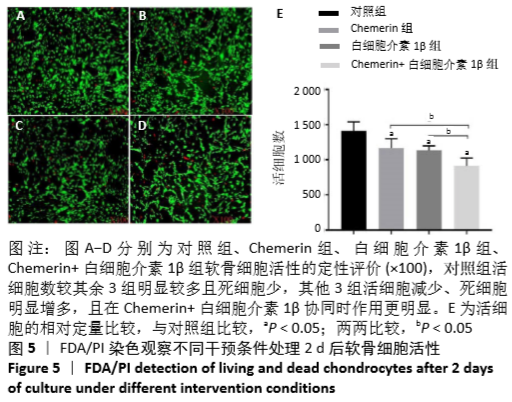
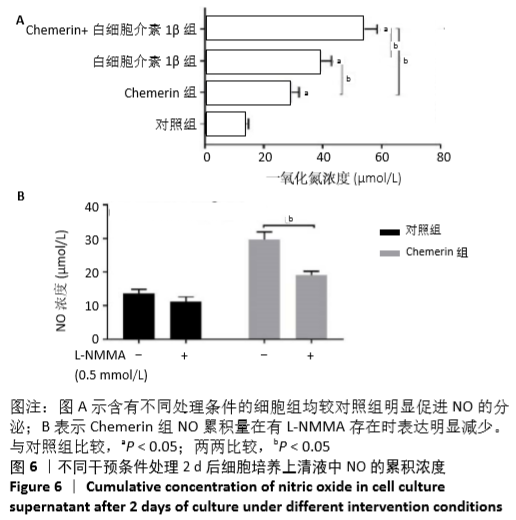
2.6 NO的浓度结果 见图6,NO在体内或水溶液中极易氧化成NO2-,在酸性条件下,NO2-与重氮盐磺酸胺生成重氮化合物,进一步与奈基乙烯基二胺偶合,产物在550 nm处有特征性吸收峰,测定其A值,可以计算NO浓度。体外药物刺激2 d后,软骨细胞中NO的生成量较对照组增加,而当Chemerin和白细胞介素1β共存时,NO的生成明显增加(图6A)。这些结果与既往的研究一致,白细胞介素1β可以促进NO的分泌,也表明Chemerin具有类似的作用。当在Chemerin中加入L-NMMA后,NO的分泌明显减少(图6B)。换句话说,L-NMMA成功地抑制了由诱导型一氧化氮合酶合成的NO,表明Chemerin通过促进软骨细胞中诱导型一氧化氮合酶表达而增加NO产生进而影响软骨细胞存活的这一过程可被L-NMMA所阻断。 "

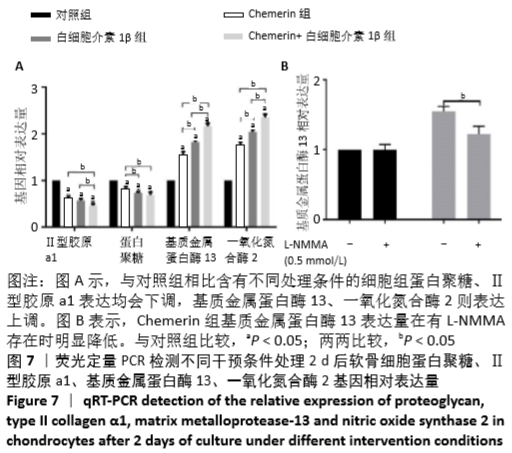
2.7 软骨细胞代谢基因分析 基因的相对表达量见图7。在培养2 d后,通过对软骨细胞蛋白聚糖(一种主要由糖胺聚糖组成的蛋白多糖)、Ⅱ型胶原a1、基质金属蛋白酶13和一氧化氮合酶2基因表达的相对定量检测,进一步研究 Chemerin对软骨细胞细胞外基质合成和降解的影响。如图7A所示,与对照组相比,3个药物实验组细胞中蛋白聚糖和Ⅱ型胶原a1类软骨形成标记基因的相对表达水平均降低,而与细胞外基质降解密切相关的基因一氧化氮合酶2和基质金属蛋白酶13的表达水平显著增加。此外,实验结果表明Chemerin的作用可能不像白细胞介素1β那样强大,但两者之间的协同作用在基因水平上也是显而易见的。在Chemerin组中加入L-NMMA后,基质金属蛋白酶13的相对表达也明显降低(图7B),与图6B的结果一致,这也反过来证明NO能促进基质金属蛋白酶13基因的表达。"
| [1] HUNTER DJ, BIERMA-ZEINSTRA S. Osteoarthritis. Lancet. 2019;393:1745-1759. [2] YOUM J, CHAN V, BELKORA J, et al. Impact of socioeconomic factors on informed decision making and treatment choice in patients with hip and knee OA.J Arthroplasty. 2015;30:171-175. [3] HUNTER DJ, SCHOFIELD D, CALLANDER E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol. 2014;10(7):437-441. [4] WANG T, HE C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018;44:38-50. [5] THIJSSEN E, VAN CAAM A, VAN DER KRAAN PM. Obesity and osteoarthritis, more than just wear and tear: pivotal roles for inflamed adipose tissue and dyslipidaemia in obesity-induced osteoarthritis. Rheumatology (Oxford). 2015;54(4):588-600. [6] FRANCISCO V, PÉREZ T, PINO J, et al. Biomechanics, obesity, and osteoarthritis. The role of adipokines: When the levee breaks. J Orthop Res. 2018;36(2):594-604. [7] SINGER SP, DAMMERER D, KRISMER M, et al. Maximum lifetime body mass index is the appropriate predictor of knee and hip osteoarthritis. Arch Orthop Trauma Surg. 2018;138(1):99-103. [8] REYES C, LEYLAND KM, PEAT G, et al. Association between overweight and obesity and risk of clinically diagnosed knee, hip, and hand osteoarthritis: a population-based cohort study. Arthritis Rheumatol. 2016;68(8):1869-1875. [9] LARRAÑAGA-VERA A, LAMUEDRA A, PÉREZ-BAOS S, et al. Increased synovial lipodystrophy induced by high fat diet aggravates synovitis in experimental osteoarthritis. Arthritis Res Ther. 2017;19(1):264. [10] XIE C, CHEN Q. Adipokines: New Therapeutic Target for Osteoarthritis? Curr Rheumatol Rep. 2019;21(12):71. [11] CHEN X, LU J, BAO J, et al. Adiponectin: a biomarker for rheumatoid arthritis?. Cytokine Growth Factor Rev. 2013;24(1):83-89. [12] RUSCITTI P, DI BENEDETTO P, BERARDICURTI O, et al. Adipocytokines in rheumatoid arthritis: the hidden link between inflammation and cardiometabolic comorbidities. J Immunol Res. 2018;2018:8410182. [13] YAN M, ZHANG J, YANG H, et al. The role of leptin in osteoarthritis. Medicine (Baltimore). 2018;97(14):e0257. [14] STOJEK M. The role of chemerin in human disease. Postepy Hig Med Dosw (Online). 2017;71:110-117. [15] 宫计划, 徐昭.2型糖尿病患者血清脂肪细胞因子Chemerin表达水平及临床价值[J].中国医学创新,2018,15(27):22-25. [16] KAUR J, MATTU HS, CHATHA K, et al. Chemerin in human cardiovascular disease. Vascul Pharmacol. 2018;110:1-6. [17] HUANG K, DU G, LI L, et al. Association of chemerin levels in synovial fluid with the severity of knee osteoarthritis. Biomarkers. 2012;17(1):16-20. [18] CIPOLLETTA C, JOUZEAU JY, GEGOUT-POTTIE P, et al. Modulation of IL-1-induced cartilage injury by NO synthase inhibitors: a comparative study with rat chondrocytes and cartilage entities. Br J Pharmacol. 1998;124(8): 1719-1727. [19] JONASON JH, HOAK D, O’KEEFE RJ. Primary murine growth plate and articular chondrocyte isolation and cell culture. Methods Mol Biol. 2015; 1226:11-18. [20] BUECHLER C, FEDER S, HABERL EM, et al. Chemerin Isoforms and Activity in Obesity. Int J Mol Sci. 2019;20(5):1128. [21] MAKRILAKIS K, FRAGIADAKI K, SMITH J, et al. Interrelated reduction of chemerin and plasminogen activator inhibitor-1 serum levels in rheumatoid arthritis after interleukin-6 receptor blockade. Clin Rheumatol. 2015;34(3):419-427. [22] 段国庆,任春凤.血清chemerin含量与膝骨关节炎严重程度的相关性[J].中华临床医师杂志(电子版),2015,9(1):63-66. [23] ZHAO L, YAMAGUCHI Y, GE X, et al. Chemerin 156F, generated by chymase cleavage of prochemerin, is elevated in joint fluids of arthritis patients. Arthritis Res Ther. 2018;20(1):132. [24] EISINGER K, BAUER S, SCHÄFFLER A, et al. Chemerin induces CCL2 and TLR4 in synovial fibroblasts of patients with rheumatoid arthritis and osteoarthritis. Exp Mol Pathol. 2012;92(1):90-96. [25] BERG V, SVEINBJÖRNSSON B, BENDIKSEN S, et al. Human articular chondrocytes express ChemR23 and chemerin; ChemR23 promotes inflammatory signalling upon binding the ligand chemerin(21-157). Arthritis Res Ther. 2010;12(6):R228. [26] MAN GS, MOLOGHIANU G. Osteoarthritis pathogenesis - a complex process that involves the entire joint. J Med Life. 2014;7(1):37-41. [27] GUILAK F, NIMS RJ, DICKS A, et al. Osteoarthritis as a disease of the cartilage pericellular matrix. Matrix Biol. 2018;71-72:40-50. [28] DERFOUL A, MIYOSHI AD, FREEMAN DE, et al. Glucosamine promotes chondrogenic phenotype in both chondrocytes and mesenchymal stem cells and inhibits MMP-13 expression and matrix degradation. Osteoarthritis Cartilage. 2007;15(6):646-655. [29] GROGAN SP, CHEN X, SOVANI S, et al. Influence of cartilage extracellular matrix molecules on cell phenotype and neocartilage formation. Tissue Eng Part A. 2014;20(1-2):264-274. [30] YAN H, SU YX, LIN XY. In vitro culture and identification of IL-1beta induced degeneration of cartilage cells in New Zealand white rabbits knee joint. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2014;34(1):81-86. [31] HALONEN KS, MONONEN ME, JURVELIN JS, et al. Importance of depth-wise distribution of collagen and proteoglycans in articular cartilage--a 3D finite element study of stresses and strains in human knee joint. J Biomech. 2013;46(6):1184-1192. [32] CHUBINSKAYA S, KUETTNER KE, COLE AA. Expression of matrix metalloproteinases in normal and damaged articular cartilage from human knee and ankle joints. Lab Invest. 1999;79(12):1669-1677. [33] RUAN G, XU J, WANG K, et al. Associations between knee structural measures, circulating inflammatory factors and MMP13 in patients with knee osteoarthritis. Osteoarthritis Cartilage. 2018;26(8):1063-1069. [34] LI Z, LIU B, ZHAO D, et al. Protective effects of Nebivolol against interleukin-1β (IL-1β)-induced type II collagen destruction mediated by matrix metalloproteinase-13 (MMP-13). Cell Stress Chaperones. 2017;22(6): 767-774. [35] CHARLIER E, RELIC B, DEROYER C, et al. Insights on Molecular Mechanisms of Chondrocytes Death in Osteoarthritis. Int J Mol Sci. 2016;17(12):2146. [36] CHELESCHI S, FIORAVANTI A, DE PALMA A, et al. Methylsulfonylmethane and mobilee prevent negative effect of IL-1β in human chondrocyte cultures via NF-κB signaling pathway. Int Immunopharmacol. 2018;65:129-139. [37] KALUNIAN KC. Current advances in therapies for osteoarthritis. Curr Opin Rheumatol. 2016;28(3):246-250. [38] JENEI-LANZL Z, MEURER A, ZAUCKE F. Interleukin-1β signaling in osteoarthritis - chondrocytes in focus. Cell Signal. 2019;53:212-223. [39] SANTORO A, CONDE J, SCOTECE M, et al. Choosing the right chondrocyte cell line: Focus on nitric oxide. J Orthop Res. 2015;33(12):1784-1788. [40] LEONIDOU A, LEPETSOS P, MINTZAS M, et al. Inducible nitric oxide synthase as a target for osteoarthritis treatment. Expert Opin Ther Targets. 2018;22(4):299-318. [41] SOROKIN A. Nitric oxide synthase and cyclooxygenase pathways: a complex interplay in cellular signaling. Curr Med Chem. 2016;23(24):2559-2578. |
| [1] | Wu Cong, Jia Quanzhong, Liu Lun. Relationship between transforming growth factor beta1 expression and chondrocyte migration in adult articular cartilage after fragmentation [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1167-1172. |
| [2] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [3] | Lin Xuchen, Zhu Hainian, Wang Zengshun, Qi Tengmin, Liu Limin, Suonan Angxiu. Effect of xanthohumol on inflammatory factors and articular cartilage in a mouse mode of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 676-681. |
| [4] | Xu Lei, Han Xiaoqiang, Zhang Jintao, Sun Haibiao. Hyaluronic acid around articular chondrocytes: production, transformation and function characteristics [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 768-773. |
| [5] | Yang Sidi, Wang Qian, Xu Nuo, Wang Ronghan, Jin Chuanqi, Lu Ying, Dong Ming. Biodentine enhances the proliferation and differentiation of osteoblasts through upregulating bone morphogenetic protein-2 [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 516-520. |
| [6] | Feng Dongfei, He Hongxu, Xie Qi, Zhang Lili, Zhou Hui, Li Wei. Selection of key genes related to biological functions and regulation pathway in periodontal reconstruction [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(2): 253-259. |
| [7] | Guan Hong, Zhang Hongbo, Shao Yan, Guo Dong, Zhang Haiyan, Cai Daozhang. PDZ domain containing 1 deficiency promotes chondrocyte senescence in osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(2): 182-189. |
| [8] | Zhu Chunhui, Zhang Yi, Song Huanghe, Liang Wenwei. Protective effect of astaxanthin on tert-butyl hydrogen peroxide-induced chondrocyte damage [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(11): 1648-1655. |
| [9] | Yu Dong, Liu Kan, Shi Zongting, Yang Xiaoxia, Liu Hengping, Zhang Qingfeng. Pathological changes of the cervical intervertebral discs and rules of migration and apoptosis in endplate chondrocytes in a rabbit model of dynamic disequilibrium [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(11): 1675-1679. |
| [10] | Li Xuan, Sun Yimin, Li Longbiao, Wang Zhenming, Yang Jing, Wang Chenglin, Ye Ling. Manufacturing of nano-modified polycaprolactone microspheres and its biological effects in dental pulp cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(10): 1530-1536. |
| [11] | Zhou Yi, Liu Xiaoyan, Xiang Bingyan. Application advantages of concentrated growth factors in the field of tissue repair and regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(10): 1631-1640. |
| [12] | Yao Yanyi, Chen Xiaoling, Zhang Min, Wang Hong, Hao Liying, Shi Chao, Zhao Bingfeng, Jin Yaqiao, Dong Wei. miR-210/YWHAG axis with adipocyte proliferation and angiogenesis after autologous fat transplantation [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(1): 59-63. |
| [13] | Liu Xiaogang, Li Tian, Zhang Duo. Effect and mechanism of the effective components of Chinese medicine on promoting the differentiation of bone marrow mesenchymal stem cells into chondrocytes [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(1): 119-124. |
| [14] | Li Zhongkang, Zheng Jiahua, Tian Yanpeng, Huang Xianghua. Latest progress and mechanisms of mesenchymal stem cells on premature ovarian failure [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(1): 141-147. |
| [15] | Feng Zhiguo, Sun Haibiao, Han Xiaoqiang. Regulation of proliferation, differentiation and apoptosis of bone-related cells by long-stranded non-coding RNA [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(1): 112-118. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||