Chinese Journal of Tissue Engineering Research ›› 2019, Vol. 23 ›› Issue (29): 4749-4756.doi: 10.3969/j.issn.2095-4344.1819
Bone marrow mesenchymal stem cells combined with scaffolds in treatment of bone defects: present and future
Chi Yulei1, Bu Xianmin2, Zha Yumei3, Wang Haibin3, Meng Chunyang3, Wu Bin1, 3
- 1Clinical Institute, Jining Medical University, Jining 272000, Shandong Province, China; 2Jining No. 1 People’s Hospital, Jining 272000, Shandong Province, China; 3Department of Orthopedics, Affiliated Hospital of Jining Medical University, Jining 272000, Shandong Province, China
-
Revised:2019-05-17Online:2019-10-18Published:2019-10-18 -
Contact:Wu Bin, MD, Associate chief physician, Clinical Institute, Jining Medical University, Jining 272000, Shandong Province, China; Department of Orthopedics, Affiliated Hospital of Jining Medical University, Jining 272000, Shandong Province, China -
About author:Chi Yulei, Master candidate, Clinical Institute, Jining Medical University, Jining 272000, Shandong Province, China; Bu Xianmin, Master, Associate chief physician, Jining No. 1 People’s Hospital, Jining 272000, Shandong Province, China. Chi Yulei and Bu Xianmin contributed equally to this work. -
Supported by:the National Natural Science Foundation of China, No. 81572205 (to MCY); Natural Science Foundation of Shandong Province, No. ZR2014CQ042 (to WB); Fund for Teachers Support of Jining Medical University, No. JYFC2018FKJ048 (to WB); Research Fund for Lin He’s Academician Workstation of New Medicine and Clinical Translation in Jining Medical University, No. JYHL2018FMS13 (to WB)
CLC Number:
Cite this article
Chi Yulei, Bu Xianmin, Zha Yumei, Wang Haibin, Meng Chunyang, Wu Bin. Bone marrow mesenchymal stem cells combined with scaffolds in treatment of bone defects: present and future[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(29): 4749-4756.
share this article
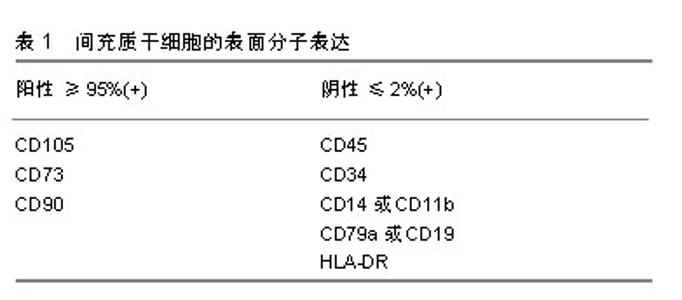
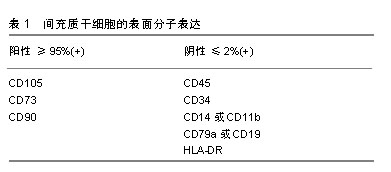
2.1 骨缺损治疗现状 骨缺损是一种严重的骨科疾病,其治疗具有挑战性。目前骨缺损的治疗技术包括骨移植技术、带血管腓骨移植技术、IIizarov骨搬运技术、Masquelet诱导膜技术、钛网cage技术、髓内钉系统骨延长技术和组织工程技术等[5-7]。移植骨可分为自体骨、同种异体骨、异种骨及人工骨4类,自体骨移植仍然是目前小缺损(<5 cm)重建的治疗标准,但由于自体骨取材有限,并容易产生医源性损伤、术后疼痛等并发症,自体骨移植的临床应用还较为局限;相比之下,同种异体骨和异种骨移植在疾病传播和免疫原性反应等方面发生的风险概率都很高,目前也并没有被接受为骨缺损的标准治疗方式。同样,Masquelet诱导膜技术和IIizarov骨搬运技术是目前较大骨缺损(>5 cm)重建的首选方法,但这些技术操作复杂,对术者技术要求高,其预后避免不了高并发症和再手术率的风险[8-10]。因此,人们不得不探索一种新的治疗方法来更好的代替自体骨。近年来,组织工程技术为治疗骨缺损提供了新的研究方向和思路,该技术将组织工程和细胞生物学的原理和方法相结合,研发相关生物材料(人工骨)复合骨髓间充质干细胞对骨缺损组织的结构、功能进行修复和改善[7],并取得了相关突破和进展。 2.2 骨髓间充质干细胞的进步 19世纪德国病理学家科恩海姆(Cohnheim)提出骨髓中存在一种非造血间充质干细胞的观点,到了19世纪中叶Friedenstein的研究证实了骨髓间充质干细胞的存在[11]。直到1991年美国生物学家卡普兰Caplan将其命名为间充质干细胞[12]。为了作为科学研究的识别标准,2006年国际细胞治疗协会(ISCT)提出了定义人类间充质干细胞的最低标准:首先,间充质干细胞在标准培养条件下保持塑料黏附性;其次,间充质干细胞必须具有特异性表面抗原的表达,见表1;第三,间充质干细胞必须在体外分化为成骨细胞、脂肪细胞和成软骨细胞[13]。根据剑桥词典,干细胞被定义为一种可以发育成任何其他类型的细胞[16]。目前,很多学者认为骨髓间充质干细胞在体内的主要功能不是多能性,而是免疫调节和营养,因此在2010年和2017年首次使用间充质干细胞一词的论文作者卡普兰Caplan呼吁更名为药用信号细胞[14-15]。还有些学者称其为间充质基质细胞,虽然名字不应该被认为是科学进步的障碍,但这些细胞临床效果的潜在预期可能会阻碍骨髓间充质干细胞在再生医学领域的作用[16]。"
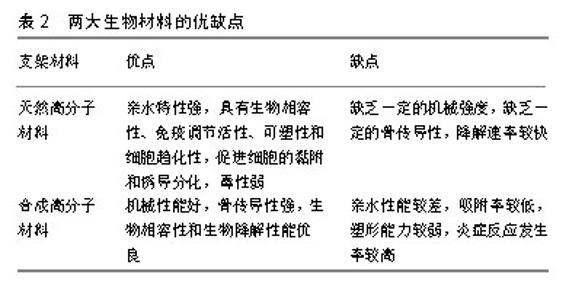
2.3 骨髓间充质干细胞的分离培养 骨髓间充质干细胞是一种很有前途的细胞,体外可分化成骨细胞、软骨细胞、脂肪细胞等[17],其数量占骨髓单核细胞的0.001%-0.01%,这一比例会随着年龄的增长而降低[18],如何高效的分离骨髓间充质干细胞是未来组织工程发展进步的前提,但直到目前为止,还没有唯一的标志物可以对其进行识别和分离[19]。骨髓间充质干细胞分离培养的方法有全骨髓贴壁法、密度梯度离心法、流式细胞仪分选法、免疫磁珠分选法、膜过滤方法及膜迁移法等[19]。流式细胞仪分选法和免疫磁珠分选法虽然能够分离出高纯度的骨髓间充质干细胞,但由于操作复杂、价格比较昂贵同时对细胞损伤较大,因此很多研究小组不把这2种方法作为首选。全骨髓贴壁法和密度梯度离心法虽然分离出骨髓间充质干细胞纯度不如以上2种方法,但操作简单、经济,是目前较为常用的分离方法,其中密度梯度离心法主要分为Ficoll[20]、Percoll[21]、Lymphoprep等离心法[22]。2016年Meppelink等[17]采用综合离心技术运用BMC装置在90 s内集中了骨髓,使单核细胞的产量提高了10倍,与Ficoll离心法相比,细胞保存率提高了2倍,这是一种既经济、高效又方便在手术室内使用的新型分离方法,将来可以为组织工程的发展打下基础。还有些学者通过适当延长细胞黏附间隔和缺氧培养的差异黏附性分离小鼠骨髓间充质干细胞,实现了一种简单易行、成本低廉的分离方法[23]。总之,骨髓间充质干细胞的分离培养还没有统一的标准,未来需要研究出更加完善的体外分离培养技术,使骨髓间充质干细胞更加高效广泛的应用于临床治疗[24]。 2.4 骨髓间充质干细胞定向分化为骨细胞机制 骨髓间充质干细胞的成骨分化是一个复杂的过程,受物理、化学及生物等因素的影响,由多种调控因子和蛋白质共同调控[25]。首先,骨祖细胞分化为前成骨细胞,然后形成成熟的成骨细胞,最后成骨细胞矿化在细胞外基质中并成为成熟的骨细胞[26]。因此,研究相关诱导因子和信号通路对于骨种植体、骨替代材料、骨再生细胞支架的发展至关重要[27]。目前,骨发育过程中重要信号通路的参与,已被各种研究证实。下面将阐述一些重要信号通路在骨折愈合修复中的作用,如Wnt/β-catenin信号通路、Notch信号通路、BMP/TGF-β通路、PI3K/Akt/mTOR通路等。 2.4.1 Wnt/β-catenin信号通路 Wnt蛋白是分泌分子家族的成员,其与七次跨膜卷曲蛋白(FZD)受体结合,导致通路的中心角色β-catenin在下游被激活[28]。Wnt信号被认为参与了骨的整个愈合过程,这种信号在细胞增殖、分化、自我更新和细胞命运的决定中起着重要作用[29]。更重要的是,Wnt信号通路参与促进成骨细胞生成和成骨分化,从而增强成骨质量。另一方面,Wnt信号抑制剂失活突变也会导致骨量增加。敲除编码可溶性FZD受体蛋白的基因,即Wnt通路的拮抗剂,在小鼠模型中会导致骨小梁矿物质的密度增高。据报道,在骨折愈合模型中,Wnt/β-catenin信号在骨折修复的不同阶段发挥的作用仍具有争议。如在骨折愈合早期,无论是抑制还是激活Wnt通路,都会抑制骨髓间充质干细胞向成骨细胞分化。后期Wnt通路会积极促进骨髓间充质干细胞成骨细胞分化[30]。 2.4.2 Notch信号通路 Notch信号通路作为一种高度进化保守的配体受体信号通路,在细胞存活、增殖及分化中发挥着重要的作用机制[31-32],Notch信号通路在Notch受体(Notch 1-4)与其配体相互作用后被激活。配体结合后,ADAM-10或-17和γ-分泌酶水解切割Notch受体,在细胞内被释放,然后转移到细胞核中以激活靶基因[31]。Notch信号通路是另一种对成骨细胞具有直接骨诱导作用的潜在途径之一。已有研究证明Notch-2受体特别是Jag-1信号通路参与骨髓间充质干细胞向成骨细胞分化。用Jag-1处理骨髓间充质干细胞会导致成骨细胞相关基因碱性磷酸酶和骨唾液蛋白表达增加,从而诱导成骨细胞生成。此外,还证明了Jag-1与生物材料结合有助于促进成骨细胞分化[33]。从小鼠胚胎肢体骨髓间充质干细胞中移除Notch-2,显著增加了青春期小鼠的小梁骨量。在间充质祖细胞中,转录因子RBPjk (RBPjk是所有典型Notch信号的中介)缺失会导致显著的高骨质量表型,其特征是成骨细胞数量增加及骨髓间充质干细胞池减少。然而,单个Notch受体的作用及其下游信号机制尚未阐明[34-35]。 2.4.3 骨形态发生蛋白/转化生长因子β通路 骨形态发生蛋白作为多功能旁分泌生长因子属于转化生长因子β家族,在成骨分化过程中起着重要作用。骨形态发生蛋白/转化生长因子β与骨形态发生蛋白或转化生长因子β特异性1型和2型丝氨酸/苏氨酸激酶受体之间的相互作用通过Smad依赖性途径和Smad非依赖性信号传导启动信号级联反应[36]。体内和体外实验都证明了在骨骼发育开始骨形态发生蛋白/转化生长因子β信号通路是不可缺少的重要信号通路[37]。此外,骨形态发生蛋白信号通路对于成骨的进展和成熟至关重要,这可能取决于作用于Runx2下游的转录因子Osterix。在骨形态发生蛋白中,骨形态发生蛋白2是研究的热点,其临床应用已经帮助了越来越多患者在治疗骨缺损领域实现了骨再生[38]。 有许多治疗策略对临床治疗骨缺损具有重要意义,这为骨再生开辟了新的途径。最有趣的策略之一是靶向上述分子信号传导途径,以通过增加成骨细胞的数量或促进其成熟来增加骨再生的功效。如PI3K/Akt/mTOR通路、MAPK通路、PDGF信号、IGF信号、FGF通路、钙离子等信号通路在细胞增殖、分化、生长、存活、发育、再生、自我更新和细胞命运的决定中起着重要作用[25-27]。另外,还有些研究小组认为旁分泌因子在骨缺损部位产生促成骨微环境,在骨再生过程中起着至关重要的作用[39-41]。总之,深入了解骨再生过程中所涉及的分子信号通路,有助于将新的治疗方法从实验带到临床。 2.5 支架材料 骨修复能力会受到骨感染或周围组织血供不足、全身疾病等多种情况的负面影响[42]。人们对骨移植物的选择仍然受到很大的限制。支架材料主要分为合成高分子材料和天然高分子材料两大类,合成高分子材料如羟基磷灰石、磷酸三钙、磷酸钙水泥、纳米陶瓷、聚乙乳酸和聚乙醇酸等[43-51],天然高分子材料如胶原、明胶和壳聚糖等[52-53],目前生物支架材料研究的热点是将不同的生物材料混合在一起,以制备高性能的复合材料,克服单一组分材料的缺陷,见表2。人们再通过纳米技术、电纺技术、低温技术、固-液相分离技术、基因工程及3D生物打印技术等制备出具有生物活性、骨传导性、骨诱导性、降解可控性等多孔生物支架[54-57]。目前研究表明,携带骨形态发生蛋白2和血管内皮生长因子基因的纳米支架材料具有诱导基因表达的功能,并能使携带基因在体内外可控性表达,进而加速修复临界大小的颅骨缺损的能力[58-59]。另外,目前3D打印生物支架和可注射性生物支架都是研究的热点。"

| [1]Majidinia M, Sadeghpour A, Yousefi B. The roles of signaling pathways in bone repair and regeneration. J Cell Physiol. 2018;233(4):2937-2948. [2]Liu Z, Yuan X, Fernandes G, et al. The combination of nano-calcium sulfate/platelet rich plasma gel scaffold with BMP2 gene-modified mesenchymal stem cells promotes bone regeneration in rat critical-sized calvarial defects. Stem Cell Res Ther. 2017;8(1):122.[3]Chu W, Gan Y, Zhuang Y, et al. Mesenchymal stem cells and porous β-tricalcium phosphate composites prepared through stem cell screen-enrich-combine(-biomaterials) circulating system for the repair of critical size bone defects in goat tibia. Stem Cell Res Ther. 2018;9(1):157.[4]Kim DK, Kim JI, Hwang TI, et al. Bioengineered Osteoinductive Broussonetia kazinoki/Silk Fibroin Composite Scaffolds for Bone Tissue Regeneration. ACS Appl Mater Interfaces. 2017;9(2):1384-1394.[5]袁昊龙,王志刚,张锴.Masquelet技术重建骨缺损研究进展[J].中华骨科杂志,2018,38(21):1330-1336. [6]胡汉,田竞,周大鹏.成人长骨大范围骨缺损治疗的研究进展[J].中国临床实用医学,2016,7(6):102-104. [7]戚晓阳,邱旭升,施鸿飞, 等.大段骨缺损的治疗进展[J].实用骨科杂志,2017,23(8):715-719.[8]Nauth A, Schemitsch E, Norris B, et al. Critical-Size Bone Defects: Is There a Consensus for Diagnosis and Treatment. J Orthop Trauma. 2018;32 Suppl 1:S7-S11.[9]Mauffrey C, Barlow BT, Smith W. Management of segmental bone defects. J Am Acad Orthop Surg. 2015;23(3):143-153.[10]Whitely M, Cereceres S, Dhavalikar P, et al. Improved in situ seeding of 3D printed scaffolds using cell-releasing hydrogels. Biomaterials. 2018;185:194-204.[11]Kfoury Y, Scadden DT. Mesenchymal cell contributions to the stem cell niche. Cell Stem Cell. 2015;16(3):239-253.[12]Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5): 641-650.[13]Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317.[14]Caplan AI. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl Med. 2017;6(6):1445-1451.[15]Caplan AI. What's in a name?Tissue Eng Part A. 2010;16(8): 2415-2417.[16]De Windt TS, Vonk LA, Saris DBF. Response to: Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl Med. 2017;6(8):1747-1748.[17]Meppelink AM, Wang XH, Bradica G, et al. Rapid isolation of bone marrow mesenchymal stromal cells using integrated centrifuge-based technology. Cytotherapy. 2016;18(6): 729-739.[18]Hoch AI, Leach JK. Concise review: optimizing expansion of bone marrow mesenchymal stem/stromal cells for clinical applications. Stem Cells Transl Med. 2015;4(4):412.[19]Nicodemou A, Danisovic L. Mesenchymal stromal/stem cell separation methods: concise review. Cell Tissue Bank. 2017; 18(4):443-460.[20]Dvorakova J, Hruba A, Velebny V, et al. Isolation and characterization of mesenchymal stem cell population entrapped in bone marrow collection sets. Cell Biol Int. 2008; 32(9):1116-1125.[21]Hung SC, Chen NJ, Hsieh SL, et al. Isolation and characterization of size-sieved stem cells from human bone marrow. Stem Cells. 2002;20(3):249-258.[22]Lunde K, Solheim S, Aakhus S, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355(12):1199-1209.[23]Hu Y, Lou B, Wu X, et al. Comparative Study on In Vitro Culture of Mouse Bone Marrow Mesenchymal Stem Cells. Stem Cells Int. 2018;2018:6704583. [24]Bara JJ, Richards RG, Alini M, et al. Concise review: Bone marrow-derived mesenchymal stem cells change phenotype following in vitro culture: implications for basic research and the clinic. Stem Cells. 2014;32(7):1713-1723.[25]Liu H, Wei LK, Jian XF, et al. Isolation, culture and induced differentiation of rabbit mesenchymal stem cells into osteoblasts. Exp Ther Med. 2018;15(4):3715-3724.[26]Huang C, Geng J, Jiang S. MicroRNAs in regulation of osteogenic differentiation of mesenchymal stem cells. Cell Tissue Res. 2017;368(2):229-238.[27]Yang H, Guo Y, Wang D, et al. Effect of TAK1 on osteogenic differentiation of mesenchymal stem cells by regulating BMP-2 via Wnt/β-catenin and MAPK pathway. Organogenesis. 2018;14(1):36-45.[28]Gruber J, Yee Z, Tolwinski NS. Developmental Drift and the Role of Wnt Signaling in Aging. Cancers (Basel). 2016;8(8)i: E73.[29]Shi J, Chi S, Xue J, et al. Emerging Role and Therapeutic Implication of Wnt Signaling Pathways in Autoimmune Diseases. J Immunol Res. 2016;2016:9392132.[30]Krishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest. 2006;116(5):1202-1209. [31]Majidinia M, Alizadeh E, Yousefi B, et al. Downregulation of Notch Signaling Pathway as an Effective Chemosensitizer for Cancer Treatment. Drug Res (Stuttg). 2016;66(11): 571-579.[32]Majidinia M, Alizadeh E, Yousefi B, et al. Co-inhibition of Notch and NF-κB Signaling Pathway Decreases Proliferation through Downregulating IκB-α and Hes-1 Expression in Human Ovarian Cancer OVCAR-3 Cells. Drug Res (Stuttg). 2017;67(1):13-19.[33]Dishowitz MI, Zhu F, Sundararaghavan HG, et al. Jagged1 immobilization to an osteoconductive polymer activates the Notch signaling pathway and induces osteogenesis. J Biomed Mater Res A. 2014;102(5):1558-1567.[34]Tu X, Chen J, Lim J, et al. Physiological notch signaling maintains bone homeostasis via RBPjk and Hey upstream of NFATc1. PLoS Genet. 2012;8(3):e1002577.[35]Wang C, Inzana JA, Mirando AJ, et al. NOTCH signaling in skeletal progenitors is critical for fracture repair. J Clin Invest. 2016;126(4):1471-1481.[36]Carreira AC, Lojudice FH, Halcsik E, et al. Bone morphogenetic proteins: facts, challenges, and future perspectives. J Dent Res. 2014;93(4):335-345.[37]Deschaseaux F, Sensébé L, Heymann D. Mechanisms of bone repair and regeneration.Trends Mol Med.2009;15(9): 417-429.[38]Schmidt-Bleek K, Willie BM, Schwabe P, et al. BMPs in bone regeneration: Less is more effective, a paradigm-shift. Cytokine Growth Factor Rev. 2016;27:141-148.[39]Li Y, Jin D, Xie W, et al. Mesenchymal Stem Cells-Derived Exosomes: A Possible Therapeutic Strategy for Osteoporosis. Curr Stem Cell Res Ther. 2018;13(5):362-368.[40]Ogata K, Katagiri W, Hibi H. Secretomes from mesenchymal stem cells participate in the regulation of osteoclastogenesis in vitro. Clin Oral Investig. 2017;21(6):1979-1988.[41]Kuroda K, Kabata T, Hayashi K, et al. The paracrine effect of adipose-derived stem cells inhibits osteoarthritis progression. BMC Musculoskelet Disord. 2015;16:236.[42]Dimitriou R, Jones E, McGonagle D, et al. Bone regeneration:current concepts and future directions.BMC Med.2011;9:66.[43]Siddiqui HA, Pickering KL, Mucalo MR. A Review on the Use of Hydroxyapatite-Carbonaceous Structure Composites in Bone Replacement Materials for Strengthening Purposes. Materials (Basel). 2018;11(10): E1813.[44]Ho-Shui-Ling A, Bolander J, Rustom LE, et al. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials. 2018;180:143-162.[45]Wang W, Yeung KWK. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact Mater. 2017;2(4):224-247.[46]Dhivya S, Keshav Narayan A, Logith Kumar R, et al. Proliferation and differentiation of mesenchymal stem cells on scaffolds containing chitosan, calcium polyphosphate and pigeonite for bone tissue engineering. Cell Prolif. 2018;51(1): e12408.[47]Ke X, Zhuang C, Yang X, et al. Enhancing the Osteogenic Capability of Core-Shell Bilayered Bioceramic Microspheres with Adjustable Biodegradation. ACS Appl Mater Interfaces. 2017;9(29):24497-24510.[48]Zhu Y, Zhang K, Zhao R, et al. Bone regeneration with micro/nano hybrid-structured biphasic calcium phosphate bioceramics at segmental bone defect and the induced immunoregulation of MSCs. Biomaterials. 2017;147:133-144.[49]Wang D, Lin Y, Chen L, et al. Guided bone regeneration using a bone tissue engineering complex consisting of a poly-dl-lactide membrane and bone mesenchymal stem cells. Oncotarget. 2017;9(23):16380-16388.[50]Kim IG, Hwang MP, Du P, et al. Bioactive cell-derived matrices combined with polymer mesh scaffold for osteogenesis and bone healing. Biomaterials. 2015;50:75-86.[51]刘相杰,宋科官.生物支架材料及间充质干细胞在骨组织工程中的研究与应用[J].中国组织工程研究,2018,22(10):1618-1624. [52]De Melo Pereira D, Habibovic P. Biomineralization-Inspired Material Design for Bone Regeneration. Adv Healthc Mater. 2018;7(22):e1800700.[53]Kozusko SD, Riccio C, Goulart M, et al. Chitosan as a Bone Scaffold Biomaterial. J Craniofac Surg. 2018;29(7):1788-1793.[54]Wang Y, Wang K, Li X, et al. 3D fabrication and characterization of phosphoric acid scaffold with a HA/β-TCP weight ratio of 60:40 for bone tissue engineering applications. PLoS One. 2017;12(4):e0174870.[55]Kutikov AB, Skelly JD, Ayers DC, et al. Templated repair of long bone defects in rats with bioactive spiral-wrapped electrospun amphiphilic polymer/hydroxyapatite scaffolds. ACS Appl Mater Interfaces. 2015;7(8):4890-4901.[56]Inzana JA, Olvera D, Fuller SM, et al. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials. 2014;35(13):4026-4034.[57]Goh YQ, Ooi CP. Fabrication and characterization of porous poly(L-lactide) scaffolds using solid-liquid phase separation. J Mater Sci Mater Med. 2008;19(6):2445-2452.[58]Raftery RM, Mencía Castaño I, Chen G, et al. Translating the role of osteogenic-angiogenic coupling in bone formation: Highly efficient chitosan-pDNA activated scaffolds can accelerate bone regeneration in critical-sized bone defects. Biomaterials. 2017;149:116-127.[59]Raftery RM, Mencía-Castaño I, Sperger S, et al. Delivery of the improved BMP-2-Advanced plasmid DNA within a gene-activated scaffold accelerates mesenchymal stem cell osteogenesis and critical size defect repair. J Control Release. 2018;283:20-31.[60]Mandrycky C, Wang Z, Kim K, et al. 3D bioprinting for engineering complex tissues. Biotechnol Adv. 2016;34(4): 422-434.[61]Melchels FP, Feijen J, Grijpma DW. A review on stereolithography and its applications in biomedical engineering. Biomaterials. 2010;31(24):6121-6130.[62]Zhang L, Yang G, Johnson BN, et al. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2019;84:16-33.[63]Chou DT, Wells D, Hong D, et al. Novel processing of iron-manganese alloy-based biomaterials by inkjet 3-D printing. Acta Biomater. 2013;9(10):8593-8603.[64]Trachtenberg JE, Placone JK, Smith BT, et al. Extrusion-based 3D printing of poly(propylene fumarate) scaffolds with hydroxyapatite gradients. J Biomater Sci Polym Ed. 2017;28(6):532-554.[65]Gudapati H, Yan J, Huang Y, et al. Alginate gelation-induced cell death during laser-assisted cell printing. Biofabrication. 2014;6(3):035022.[66]Ozbolat IT, Yu Y. Bioprinting toward organ fabrication: challenges and future trends. IEEE Trans Biomed Eng. 2013; 60(3):691-699.[67]Yan Y, Chen H, Zhang H, et al. Vascularized 3D printed scaffolds for promoting bone regeneration. Biomaterials. 2019;190-191:97-110.[68]Vo TN, Shah SR, Lu S, et al. Injectable dual-gelling cell-laden composite hydrogels for bone tissue engineering. Biomaterials. 2016;83:1-11.[69]Chen Y, Liu X, Liu R, et al. Zero-order controlled release of BMP2-derived peptide P24 from the chitosan scaffold by chemical grafting modification technique for promotion of osteogenesis in vitro and enhancement of bone repair in vivo. Theranostics. 2017;7(5):1072-1087. [70]Cui ZK, Kim S, Baljon JJ, et al. Design and Characterization of a Therapeutic Non-phospholipid Liposomal Nanocarrier with Osteoinductive Characteristics To Promote Bone Formation. ACS Nano. 2017;11(8):8055-8063.[71]Killington K, Mafi R, Mafi P, et al. A Systematic Review of Clinical Studies Investigating Mesenchymal Stem Cells for Fracture Non-Union and Bone Defects. Curr Stem Cell Res Ther. 2018;13(4):284-291.[72]Donneys A, Nelson NS, Page EE, et al. Targeting angiogenesis as a therapeutic means to reinforce osteocyte survival and prevent nonunions in the aftermath of radiotherapy. Head Neck. 2015;37(9):1261-1267.[73]Xu WL, Ong HS, Zhu Y, et al. In Situ Release of VEGF Enhances Osteogenesis in 3D Porous Scaffolds Engineered with Osterix-Modified Adipose-Derived Stem Cells. Tissue Eng Part A. 2017;23(9-10):445-457.[74]Yu WL, Sun TW, Qi C, et al. Enhanced osteogenesis and angiogenesis by mesoporous hydroxyapatite microspheres-derived simvastatin sustained release system for superior bone regeneration. Sci Rep. 2017;7:44129.[75]Wang T, Zhai Y, Nuzzo M, et al. Layer-by-layer nanofiber-enabled engineering of biomimetic periosteum for bone repair and reconstruction. Biomaterials.2018;182: 279-288.[76]Ko E, Lee JS, Kim H, et al. Electrospun Silk Fibroin Nanofibrous Scaffolds with Two-Stage Hydroxyapatite Functionalization for Enhancing the Osteogenic Differentiation of Human Adipose-Derived Mesenchymal Stem Cells. ACS Appl Mater Interfaces. 2018;10(9): 7614-7625.[77]Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326(5957):1216-1219.[78]Hu H, Zhao P, Liu J, et al. Lanthanum phosphate/chitosan scaffolds enhance cytocompatibility and osteogenic efficiency via the Wnt/β-catenin pathway. J Nanobiotechnology. 2018; 16(1):98.[79]A A, Menon D, T B S, et al. Bioinspired Composite Matrix Containing Hydroxyapatite-Silica Core-Shell Nanorods for Bone Tissue Engineering. ACS Appl Mater Interfaces. 2017;9(32):26707-26718.[80]Aquino-Martínez R, Angelo AP, Pujol FV. Et al. Calcium-containing scaffolds induce bone regeneration by regulating mesenchymal stem cell differentiation and migration. Stem Cell Res Ther. 2017;8(1):265.[81]Cheng CW, Solorio LD, Alsberg E. Decellularized tissue and cell-derived extracellular matrices as scaffolds for orthopaedic tissue engineering. Biotechnol Adv. 2014;32(2):462-484.[82]Lu H, Hoshiba T, Kawazoe N, et al. Cultured cell-derived extracellular matrix scaffolds for tissue engineering. Biomaterials. 2011;32(36):9658-9666.[83]Kim B, Ventura R, Lee BT. Functionalization of porous BCP scaffold by generating cell-derived extracellular matrix from rat bone marrow stem cells culture for bone tissue engineering. J Tissue Eng Regen Med. 2018;12(2): e1256-e1267.[84]Harvestine JN, Vollmer NL, Ho SS, et al. Extracellular Matrix-Coated Composite Scaffolds Promote Mesenchymal Stem Cell Persistence and Osteogenesis. Biomacromolecules. 2016;17(11):3524-3531.[85]Yan Y, Cheng B, Chen K, et al. Enhanced Osteogenesis of Bone Marrow-Derived Mesenchymal Stem Cells by a Functionalized Silk Fibroin Hydrogel for Bone Defect Repair. Adv Healthc Mater. 2019;8(3):e1801043.[86]Ho CY, Sanghani A, Hua J, et al. Mesenchymal stem cells with increased stromal cell-derived factor 1 expression enhanced fracture healing. Tissue Eng Part A. 2015;21(3-4): 594-602.[87]Song JE, Tripathy N, Lee DH, et al. Quercetin Inlaid Silk Fibroin/Hydroxyapatite Scaffold Promotes Enhanced Osteogenesis. ACS Appl Mater Interfaces. 2018;10(39): 32955-32964.[88]Wu S, Yu Q, Sun Y, et al. Synergistic effect of a LPEMF and SPIONs on BMMSC proliferation, directional migration, and osteoblastogenesis. Am J Transl Res. 2018;10(5):1431-1443.[89]Šponer P, Filip S, Ku?era T, et al. Utilizing Autologous Multipotent Mesenchymal Stromal Cells and β-Tricalcium Phosphate Scaffold in Human Bone Defects: A Prospective, Controlled Feasibility Trial. Biomed Res Int. 2016;2016: 2076061.[90]Gjerde C, Mustafa K, Hellem S, et al. Cell therapy induced regeneration of severely atrophied mandibular bone in a clinical trial. Stem Cell Res Ther. 2018;9(1):213.[91]Quarto R, Mastrogiacomo M, Cancedda R, et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344(5):385-386.[92]Hibi H, Yamada Y, Ueda M, et al. Alveolar cleft osteoplasty using tissue-engineered osteogenic material. Int J Oral Maxillofac Surg. 2006;35(6):551-555.[93]Shayesteh YS, Khojasteh A, Soleimani M, et al. Sinus augmentation using human mesenchymal stem cells loaded into a beta-tricalcium phosphate/hydroxyapatite scaffold. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106(2): 203-209.[94]Meijer GJ, de Bruijn JD, Koole R, et al. Cell based bone tissue engineering in jaw defects. Biomaterials. 2008;29(21): 3053-3061.[95]Redondo LM, García V, Peral B, et al. Repair of maxillary cystic bone defects with mesenchymal stem cells seeded on a cross-linked serum scaffold. J Craniomaxillofac Surg. 2018; 46(2):222-229. |
| [1] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [2] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [3] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [4] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [5] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| [6] | Pei Lili, Sun Guicai, Wang Di. Salvianolic acid B inhibits oxidative damage of bone marrow mesenchymal stem cells and promotes differentiation into cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1032-1036. |
| [7] | Wang Shiqi, Zhang Jinsheng. Effects of Chinese medicine on proliferation, differentiation and aging of bone marrow mesenchymal stem cells regulating ischemia-hypoxia microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1129-1134. |
| [8] | Hua Haotian, Zhao Wenyu, Zhang Lei, Bai Wenbo, Wang Xinwei. Meta-analysis of clinical efficacy and safety of antibiotic artificial bone in the treatment of chronic osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 970-976. |
| [9] | Zhang Bin, Sun Lihua, Zhang Junhua, Liu Yusan, Cui Caiyun. A modified flap immediate implant is beneficial to soft tissue reconstruction in maxillary aesthetic area [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 707-712. |
| [10] | Liu Bo, Chen Xianghe, Yang Kang, Yu Huilin, Lu Pengcheng. Mechanism of DNA methylation in exercise intervention for osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 791-797. |
| [11] | Chen Junyi, Wang Ning, Peng Chengfei, Zhu Lunjing, Duan Jiangtao, Wang Ye, Bei Chaoyong. Decalcified bone matrix and lentivirus-mediated silencing of P75 neurotrophin receptor transfected bone marrow mesenchymal stem cells to construct tissue-engineered bone [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 510-515. |
| [12] | Li Chenjie, Lü Linwei, Song Yang, Liu Jingna, Zhang Chunqiu. Measurement and statistical analysis of trabecular morphological parameters of titanium alloy peri-prosthesis under preload [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 516-520. |
| [13] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [14] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [15] | Ye Haimin, Ding Linghua, Kong Weihao, Huang Zutai, Xiong Long. Role and mechanism of hierarchical microchanneled bone scaffolds in promoting osteogenesis and angiogenesis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 621-625. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||