Chinese Journal of Tissue Engineering Research ›› 2019, Vol. 23 ›› Issue (29): 4743-4748.doi: 10.3969/j.issn.2095-4344.1797
Previous Articles Next Articles
Conditioned medium from cultured mesenchymal stem cells: a potential for repairing multiple diseases and injuries
Qiu Jiling1, 2, Wang Xiaotong1, 2, Zhou Haowen1, 2, Yang Pishan1, 2, Song Aimei1, 2
- 1Shandong Provincial Key Laboratory of Oral Tissue Regeneration, Jinan 250100, Shandong Province, China; 2School of Stomatology, Shandong University, Jinan 250012, Shandong Province, China
-
Revised:2019-04-15Online:2019-10-18Published:2019-10-18 -
Contact:Song Aimei, Master’s supervisor, Shandong Provincial Key Laboratory of Oral Tissue Regeneration, Jinan 250100, Shandong Province, China; School of Stomatology, Shandong University, Jinan 250012, Shandong Province, China -
About author:Qiu Jiling, Master candidate, Shandong Provincial Key Laboratory of Oral Tissue Regeneration, Jinan 250100, Shandong Province, China; School of Stomatology, Shandong University, Jinan 250012, Shandong Province, China -
Supported by:the National Natural Science Foundation of China, No. 81771076 (to YPS)
CLC Number:
Cite this article
Qiu Jiling, Wang Xiaotong, Zhou Haowen, Yang Pishan, Song Aimei. Conditioned medium from cultured mesenchymal stem cells: a potential for repairing multiple diseases and injuries [J]. Chinese Journal of Tissue Engineering Research, 2019, 23(29): 4743-4748.
share this article
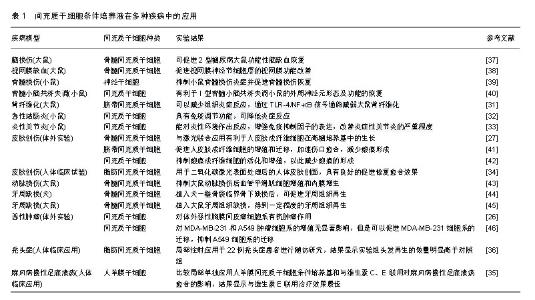
2.1 间充质干细胞条件培养液的成分 间充质干细胞在生长过程中会将一些活性物质分泌到培养环境中,这些活性物质会影响其他细胞的生长和/或功能,在创伤修复和内环境稳态的各个方面发挥重要作用,这称为间充质干细胞的旁分泌功能[6]。相关研究多在维持干细胞干性的培养过程中收集条件培养液,而对于干细胞接受诱导培养后定向分化过程中收集的条件培养液少见报道。因此,该综述所指的条件培养液,除非特别说明,均指的是维持间充质干细胞干性及生长的基础培养液。Engel等[7]研究表明干细胞的旁分泌物由分泌蛋白(如生长因子、血管生成因子、激素、细胞因子、细胞外基质蛋白、细胞外基质蛋白酶和激素)和遗传物质(如双链DNA、mRNA、microRNA和lncRNA)组成,通过细胞外囊泡或外泌体释放。Pawitan等[8]获取的干细胞条件培养液中通常含有多种活性物质,条件培养液的主要成分有:①生长因子,包括血管内皮生长因子、血小板衍生生长因子、胰岛素样生长因子、肝细胞生长因子、碱性成纤维细胞生长因子等;②炎性因子,包括转化生长因子β和一些白细胞介素,例如白细胞介素6、白细胞介素10、白细胞介素27、白细胞介素17E、白细胞介素13、白细胞介素12p70、白细胞介素8、白细胞介素9、白细胞介素1β等;③细胞外基质蛋白和组织重塑酶等,包括血管生成素、集落刺激因子、单核细胞趋化蛋白1,金属蛋白酶组织抑制剂1、丝氨酸蛋白酶抑制剂E1、尿激酶型纤溶酶原激活物等。 虽然同是间充质干细胞,但由于组织来源不同,可能会受到特异组织来源的影响而导致某些特异因子的表达不同,包括种类和数量。例如Inukai等[9]采用ELISA法检测人骨髓间充质干细胞条件培养液(常氧培养48 h)中胰岛素样生长因子1、血管内皮生长因子、转化生长因子β1、肝细胞生长因子水平分别为(1 515.6±211.8) ng/L,(465.8±108.8) ng/L,(339.8±14.4) ng/L和(20.3±7.9) ng/L,而未检测到碱性成纤维细胞生长因子、血小板衍生生长因子BB、骨形态发生蛋白2和基质细胞衍生因子1。然而,Kim等[10]在常氧下培养48 h采用ELISA法检测到人脐血间充质干细胞中表皮生长因子、血管内皮生长因子、粒细胞集落刺激因子、粒细胞-巨噬细胞集落刺激因子水平分别为(3 286±419) ng/L,(2 463±151) ng/L,(3 615±173) ng/L,(3 623±345) ng/L,碱性成纤维细胞生长因子、角化细胞生长因子、转化生长因子β1、血小板衍生生长因子水平与人脐血内皮细胞条件培养液中含量相近。 即使来自同一种类干细胞,条件培养液所含成分还会随培养条件的不同而发生变化。Song等[11]研究将低氧和常氧条件下得到的间充质干细胞条件培养液进行比较,检测到66种蛋白质表达差异,其中2种原肌球蛋白亚型在低氧条件培养液中显著增高,进一步分析表明这些蛋白质大多与心脏调节有关。Overath等[12]研究发现脂肪干细胞经过低氧处理(0.5%),条件培养液中有68种蛋白表达增加了2倍以上,从而显著提升了脂肪干细胞条件培养液对顺铂诱导大鼠急性肺损伤的治疗效果。Jiang等[13]研究表明,与常氧培养获得的骨髓间充质干细胞条件培养液比较,经过低氧预处理的骨髓间充质干细胞条件培养液中有14种蛋白明显增加,低氧预处理骨髓间充质干细胞条件培养液在减少大鼠缺血性脑卒中后脑组织损伤和改善神经系统恢复方面起着至关重要的作用。因此,研究者可根据需要调整干细胞的培养环境,引导干细胞分泌目标因子,从而更好地将干细胞条件培养液用于疾病的治疗。 2.2 间充质干细胞条件培养液的作用 骨髓间充质干细胞是较早培养和研究较多的间充质干细胞,骨髓间充质干细胞参与修复组织损伤过程中可能至少有2种不同的作用方式:旁分泌机制以及分化替代机制[14]。干细胞的旁分泌作用包括趋化性能、免疫调节功能、细胞生长支持功能、抗细胞凋亡及血管生成功能等,是通过干细胞分泌的生长因子等实现的。干细胞条件培养液还具有促进其他细胞向干细胞方向转化以及使其保持干细胞活性等功能。 2.2.1 趋化性能 多项体外研究表明干细胞条件培养液能影响内皮细胞、角化细胞、成纤维细胞的增殖和/或迁移[15-16]。Chen等[17]研究表明大鼠骨髓间充质干细胞条件培养液可以促进肌腱细胞的增殖和迁移。Ando等[18]研究表明间充质干细胞条件培养液中所包含的因子可以募集小鼠骨髓基质细胞和内皮细胞/内皮祖细胞,起到抑制炎症和细胞凋亡,促进成骨细胞分化、血管生成和细胞增殖的作用,并认为单核细胞趋化因子1/3和白细胞介素3/6是募集小鼠骨髓基质细胞和内皮细胞/内皮祖细胞的关键因素。 2.2.2 免疫调节功能 Nagata等[19]研究表明,牙周膜干细胞条件培养液能降低牙周组织中肿瘤坏死因子α mRNA水平,抑制单核巨噬细胞原始细胞群体中肿瘤坏死因子α mRNA水平,同时降低白细胞介素1水平,提高白细胞介素10水平,通过抑制炎症反应、调节免疫,从而起到促进牙周再生的作用。白海等[20]研究表明骨髓间充质干细胞条件培养液可能通过影响T淋巴细胞分泌γ-干扰素和白细胞介素4进而抑制异体外周血T淋巴细胞增殖。 2.2.3 血管生成、细胞生长支持 刘新宾等[21]研究表明间充质干细胞条件培养液中含有多种与血管生成、组织修复、细胞存活和抗炎作用相关联的生物活性物质,例如肿瘤坏死因子α、碱性成纤维细胞生长因子2、转化生长因子β、白细胞介素1、白细胞介素6等细胞因子,在胶原合成和血管生成、促进心肌细胞肥大、代偿心功能以及心肌重塑作用中起到了重要作用。 2.2.4 保持干细胞活性或促进其他细胞转化为干细胞 Qi等[22]研究表明脐带间充质干细胞条件培养液能使高糖诱导的髓核间充质干细胞Ⅱ型胶原蛋白和蛋白多聚糖表达升高,从而减轻细胞外基质降解,缓解高糖诱导的干细胞凋亡。Hu等[23]研究表明,骨髓间充质干细胞条件培养液可以诱导外周血单核细胞获得间充质干细胞的特征,为临床实践中获得间充质干细胞提供了新的途径。 2.3 间充质干细胞条件培养液的制取 现有众多研究表明,即使是对于同一种间充质干细胞来源的条件培养液,不同研究所采取的制取标准等也有所不同,例如干细胞的数量、代数、培养基种类、培养条件以及条件培养液的进一步加工,尚未达成一致。 不同的研究使用的基础培养基种类也不尽相同,还可能采用完全培养基或无血清培养基,制取条件培养液所选用的培养时间从6 h到5 d不等,其中24 h或48 h比较常见,Ando等[18]将间充质干细胞采用完全培养基培养至融合度70%-80%时,更换无血清培养基,48 h后收集上清液用于大鼠实验,结果显示间充质干细胞条件培养液对牵张成骨有促进作用。 不同研究所选取的干细胞培养条件也可能不同。例如一些研究在制取干细胞条件培养液时对细胞进行常氧(氧体积分数为21%)培养,一些研究则选取不同的低氧条件(氧体积分数为0.5%,1%,1.5%,2%等)。Jiang等[24]研究表明低氧使骨髓间充质干细胞条件培养液中血管内皮生长因子、肝细胞生长因子、血小板衍生生长因子等表达增加,从而促进骨髓间充质干细胞的增殖和干性维持,并且增强其促血管生成特性。 根据研究目的不同,研究者会对获得的条件培养液进行进一步加工,例如浓缩得到不同浓度的干细胞条件培养液。一般来说,体外研究使用的是非浓缩的干细胞条件培养液,体内研究常使用干细胞条件培养液的浓缩液。Nagata等[19]使用离心机以及切断界点为10 kD的超滤离心管获得不同浓缩倍数的条件培养液,植入大鼠牙周骨缺损,结果显示低浓度条件培养液没有明显作用,高浓度条件培养液组可观察到明显的牙周组织再生。 2.4 间充质干细胞条件培养液在组织再生中的应用 移植间充质干细胞条件培养液已经在一些体外研究、多种动物创伤及疾病的组织再生模型中取得较好效果,例如心肌梗死、脑损伤、脑缺血、脊髓损伤、骨缺损、肾纤维化、结肠炎、关节炎、牙周缺损等。人体相关临床应用虽较少,但也已有报道。 2.4.1 体外实验研究 Lee等[25]研究显示间充质干细胞条件培养液可以诱导人胚胎干细胞和多能干细胞的成骨及成软骨方向分化。Cortes-Dericks等[26]研究表明人肺源性间充质干细胞条件培养液对体外恶性胸膜间皮瘤细胞系有抗癌作用。Hendudari等[27]发现联合骨髓间充质干细胞条件培养液与激光有利于人皮肤成纤维细胞在高糖培养基中的生长,为研究皮肤成纤维细胞特性提供了实验方法。 2.4.2 动物体内研究 He等[28]研究表明低氧诱导下脂肪间充质干细胞条件培养液可以促进心肌细胞增殖和迁移,有利于大鼠心肌梗死的恢复;Faezi等[29]研究表明间充质干细胞条件培养液可以促进大鼠局灶脑缺血功能性恢复;Szekiova等[30]研究表明大鼠脂肪间充质干细胞条件培养液在体外模拟局部炎症的脊髓损伤模型中可以对神经元起到保护作用;Liu等[31]研究发现人脐带间充质干细胞条件培养液可以减少组织炎症反应,通过TLR-4/NF-κB信号通路减弱大鼠肾纤维化;Pouya等[32]研究发现将间充质干细胞条件培养液注射应用于小鼠急性结肠炎具有免疫调节功能,可降低炎症反应;Kay等[33]研究表明间充质干细胞条件培养液能改善小鼠炎症性关节炎的严重程度。Nagata等[19]将牙周膜干细胞条件培养液植入大鼠牙周组织缺损,获得了较好的牙周组织再生,并且缺损的再生效果与条件培养液的浓度显著相关,进一步研究表明牙周膜干细胞条件培养液是通过抑制牙周创区的炎症因子,主要是肿瘤坏死因子α,从而促进牙周组织再生。 2.4.3 人体临床应用 Zhou等[34]研究表明脂肪间充质干细胞条件培养液用于二氧化碳激光表面处理后的人体皮肤创面,具有良好的促进修复愈合效果;Prakoeswa等[35]研究比较局部单独应用人羊膜间充质干细胞条件培养基以及和维生素C、E联合对麻风病慢性足底溃疡愈合的影响,结果显示与维生素E联合治疗效果最佳;Fukuoka等[36]研究表明脂肪间充质干细胞条件培养液局部注射于22例秃头症患者进行随访研究,结果显示实验组头发再生的数量明显高于对照组,因此局部注射条件培养液有望成为毛发再生的一种新技术。 综上所述,间充质干细胞条件培养液已在多种疾病中取得了显著效果,具体详见表1[26-27,31-46]。"

| [1]Mundra V, Gerling IC, Mahato RI. Mesenchymal stem cell-based therapy. Mol Pharm. 2013;10(1):77-89.[2]Balaji S, Keswani SG, Crombleholme TM. The Role of Mesenchymal Stem Cells in the Regenerative Wound Healing Phenotype. Adv Wound Care (New Rochelle). 2012;1(4): 159-165.[3]von Bahr L, Batsis I, Moll G, et al. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells. 2012;30(7):1575-1578.[4]Kim HO, Choi SM, Kim HS. Mesenchymal stem cell derived secretome and macrovesicles as a cell free therapeutics for neurodegenerative disorders. Tissue Eng Regen Med. 2013; 10(3):93-101.[5]Katagiri W, Osugi M, Kawai T, et al. First-in-human study and clinical case reports of the alveolar bone regeneration with the secretome from human mesenchymal stem cells. Head Face Med. 2016;12:5.[6]Ulivi V, Tasso R, Cancedda R, et al. Mesenchymal stem cell paracrine activity is modulated by platelet lysate: induction of an inflammatory response and secretion of factors maintaining macrophages in a proinflammatory phenotype. Stem Cells Dev. 2014;23(16):1858-1869.[7]Engel FB . Stem Cell Secretome and Paracrine Activity[M]// Stem Cells and Cardiac Regeneration[A]. Springer International Publishing, 2016.[8]Pawitan JA. Prospect of stem cell conditioned medium in regenerative medicine. Biomed Res Int. 2014;2014:965849.[9]Inukai T, Katagiri W, Yoshimi R, et al. Novel application of stem cell-derived factors for periodontal regeneration. Biochem Biophys Res Commun. 2013;430(2):763-768.[10]Kim J, Lee JH, Yeo SM, et al. Stem cell recruitment factors secreted from cord blood-derived stem cells that are not secreted from mature endothelial cells enhance wound healing. In Vitro Cell Dev Biol Anim. 2014;50(2):146-154.[11]Song SW, Kim KE, Choi JW, et al. Proteomic Analysis and Identification of Paracrine Factors in Mesenchymal Stem Cell-Conditioned Media under Hypoxia. Cell Physiol Biochem. 2016;40(1-2):400-410.[12]Overath JM, Gauer S, Obermüller N, et al. Short-term preconditioning enhances the therapeutic potential of adipose- derived stromal/stem cell-conditioned medium in cisplatin-induced acute kidney injury. Exp Cell Res. 2016; 342(2):175-183.[13]Jiang RH, Wu CJ, Xu XQ, et al. Hypoxic conditioned medium derived from bone marrow mesenchymal stromal cells protects against ischemic stroke in rats. J Cell Physiol. 2019;234(2): 1354-1368.[14]Wu Y, Wang J, Scott PG, et al. Bone marrow-derived stem cells in wound healing: a review. Wound Repair Regen. 2007; 15 Suppl 1:S18-26.[15]Li M, Zhao Y, Hao H, et al. Mesenchymal stem cell- conditioned medium improves the proliferation and migration of keratinocytes in a diabetes-like microenvironment. Int J Low Extrem Wounds. 2015;14(1): 73-86.[16]Chen J, Li Y, Hao H, et al. Mesenchymal Stem Cell Conditioned Medium Promotes Proliferation and Migration of Alveolar Epithelial Cells under Septic Conditions In Vitro via the JNK-P38 Signaling Pathway. Cell Physiol Biochem. 2015; 37(5):1830-1846.[17]Chen Q, Liang Q, Zhuang W, et al. Tenocyte proliferation and migration promoted by rat bone marrow mesenchymal stem cell- derived conditioned medium. Biotechnol Lett. 2018; 40(1): 215-224.[18]Ando Y, Matsubara K, Ishikawa J, et al. Stem cell-conditioned medium accelerates distraction osteogenesis through multiple regenerative mechanisms. Bone. 2014;61:82-90.[19]Nagata M, Iwasaki K, Akazawa K, et al. Conditioned Medium from Periodontal Ligament Stem Cells Enhances Periodontal Regeneration. Tissue Eng Part A. 2017;23(9-10):367-377.[20]白海,吴冰,王存邦,等.人骨髓间充质干细胞及条件培养液对异体T淋巴细胞分泌功能的影响[J].中国组织工程研究, 2012,16(23): 4204-4208.[21]刘新宾,张红超,郭子宽.骨髓间充质干细胞条件培养液对H2O2损伤大鼠心肌细胞的保护[J].中国组织工程研究,2012,16(1): 22-26.[22]Qi L, Wang R, Shi Q, et al. Umbilical cord mesenchymal stem cell conditioned medium restored the expression of collagen II and aggrecan in nucleus pulposus mesenchymal stem cells exposed to high glucose. J Bone Miner Metab. 2018 Sep 5. doi: 10.1007/s00774-018-0953-9. [Epub ahead of print][23]Hu G, Xu JJ, Deng ZH, et al. Supernatant of bone marrow mesenchymal stromal cells induces peripheral blood mononuclear cells possessing mesenchymal features. Int J Biol Sci. 2011;7(3):364-375.[24]Jiang CM, Liu J, Zhao JY, et al. Effects of hypoxia on the immunomodulatory properties of human gingiva-derived mesenchymal stem cells. J Dent Res. 2015;94(1):69-77.[25]Lee TJ, Jang J, Kang S, et al. Mesenchymal stem cell-conditioned medium enhances osteogenic and chondrogenic differentiation of human embryonic stem cells and human induced pluripotent stem cells by mesodermal lineage induction. Tissue Eng Part A. 2014;20(7-8): 1306-1313.[26]Cortes-Dericks L, Froment L, Kocher G, et al. Human lung-derived mesenchymal stem cell-conditioned medium exerts in vitro antitumor effects in malignant pleural mesothelioma cell lines. Stem Cell Res Ther. 2016;7:25.[27]Hendudari F, Piryaei A, Hassani SN, et al. Combined effects of low-level laser therapy and human bone marrow mesenchymal stem cell conditioned medium on viability of human dermal fibroblasts cultured in a high-glucose medium. Lasers Med Sci. 2016;31(4):749-757.[28]He J, Cai Y, Luo LM, et al. Hypoxic adipose mesenchymal stem cells derived conditioned medium protects myocardial infarct in rat. Eur Rev Med Pharmacol Sci. 2015;19(22): 4397-4406.[29]Faezi M, Nasseri Maleki S, Aboutaleb N, et al. The membrane mesenchymal stem cell derived conditioned medium exerts neuroprotection against focal cerebral ischemia by targeting apoptosis. J Chem Neuroanat. 2018;94:21-31.[30]Szekiova E, Slovinska L, Blasko J, et al. The neuroprotective effect of rat adipose tissue-derived mesenchymal stem cell-conditioned medium on cortical neurons using an in vitro model of SCI inflammation. Neurol Res. 2018;40(4):258-267.[31]Liu B, Ding F, Hu D, et al. Human umbilical cord mesenchymal stem cell conditioned medium attenuates renal fibrosis by reducing inflammation and epithelial-to- mesenchymal transition via the TLR4/NF-κB signaling pathway in vivo and in vitro. Stem Cell Res Ther. 2018;9(1):7.[32]Pouya S, Heidari M, Baghaei K, et al. Study the effects of mesenchymal stem cell conditioned medium injection in mouse model of acute colitis. Int Immunopharmacol. 2018;54:86-94.[33]Kay AG, Long G, Tyler G, et al. Mesenchymal Stem Cell- Conditioned Medium Reduces Disease Severity and Immune Responses in Inflammatory Arthritis. Sci Rep. 2017;7(1):18019.[34]Zhou BR, Xu Y, Guo SL, et al. The effect of conditioned media of adipose-derived stem cells on wound healing after ablative fractional carbon dioxide laser resurfacing. Biomed Res Int. 2013;2013:519126.[35]Prakoeswa CRS, Natallya FR, Harnindya D, et al. The efficacy of topical human amniotic membrane-mesenchymal stem cell-conditioned medium (hAMMSC-CM) and a mixture of topical hAMMSC-CM + vitamin C and hAMMSC-CM + vitamin E on chronic plantar ulcers in leprosy:a randomized control trial. J Dermatolog Treat. 2018;29(8):835-840.[36]Fukuoka H, Suga H. Hair Regeneration Treatment Using Adipose-Derived Stem Cell Conditioned Medium: Follow-up With Trichograms. Eplasty. 2015;15:e10. [37]Xiang J, Hu J, Shen T, et al. Bone marrow mesenchymal stem cells-conditioned medium enhances vascular remodeling after stroke in type 2 diabetic rats. Neurosci Lett. 2017;644: 62-66.[38]Dreixler JC, Poston JN, Balyasnikova I, et al. Delayed administration of bone marrow mesenchymal stem cell conditioned medium significantly improves outcome after retinal ischemia in rats. Invest Ophthalmol Vis Sci. 2014;55(6): 3785-3796.[39]Cheng Z, Bosco DB, Sun L, et al. Neural Stem Cell-Conditioned Medium Suppresses Inflammation and Promotes Spinal Cord Injury Recovery. Cell Transplant. 2017;26(3):469-482.[40]Suto N, Mieda T, Iizuka A, et al. Morphological and Functional Attenuation of Degeneration of Peripheral Neurons by Mesenchymal Stem Cell-Conditioned Medium in Spinocerebellar Ataxia Type 1-Knock-in Mice. CNS Neurosci Ther. 2016;22(8):670-676.[41]Li M, Luan F, Zhao Y, et al. Mesenchymal stem cell-conditioned medium accelerates wound healing with fewer scars. Int Wound J. 2017;14(1):64-73.[42]Sato C, Yamamoto Y, Funayama E, et al. Conditioned Medium Obtained from Amnion-Derived Mesenchymal Stem Cell Culture Prevents Activation of Keloid Fibroblasts. Plast Reconstr Surg. 2018;141(2):390-398.[43]Iso Y, Usui S, Toyoda M, et al. Bone marrow-derived mesenchymal stem cells inhibit vascular smooth muscle cell proliferation and neointimal hyperplasia after arterial injury in rats. Biochem Biophys Rep. 2018;16:79-87.[44]Inukai T, Katagiri W, Yoshimi R, et al. Novel application of stem cell-derived factors for periodontal regeneration. Biochem Biophys Res Commun. 2013;430(2):763-768.[45]Kawai T, Katagiri W, Osugi M, et al. Secretomes from bone marrow-derived mesenchymal stromal cells enhance periodontal tissue regeneration. Cytotherapy. 2015;17(4): 369-381.[46]Li P, Zhou H, Di G, et al. Mesenchymal stem cell-conditioned medium promotes MDA-MB-231 cell migration and inhibits A549 cell migration by regulating insulin receptor and human epidermal growth factor receptor 3 phosphorylation. Oncol Lett. 2017;13(3):1581-1586. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [3] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [4] | Shi Yangyang, Qin Yingfei, Wu Fuling, He Xiao, Zhang Xuejing. Pretreatment of placental mesenchymal stem cells to prevent bronchiolitis in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 991-995. |
| [5] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [6] | Fan Quanbao, Luo Huina, Wang Bingyun, Chen Shengfeng, Cui Lianxu, Jiang Wenkang, Zhao Mingming, Wang Jingjing, Luo Dongzhang, Chen Zhisheng, Bai Yinshan, Liu Canying, Zhang Hui. Biological characteristics of canine adipose-derived mesenchymal stem cells cultured in hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1002-1007. |
| [7] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [8] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [9] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| [10] | Duan Liyun, Cao Xiaocang. Human placenta mesenchymal stem cells-derived extracellular vesicles regulate collagen deposition in intestinal mucosa of mice with colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1026-1031. |
| [11] | Pei Lili, Sun Guicai, Wang Di. Salvianolic acid B inhibits oxidative damage of bone marrow mesenchymal stem cells and promotes differentiation into cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1032-1036. |
| [12] | Wang Xianyao, Guan Yalin, Liu Zhongshan. Strategies for improving the therapeutic efficacy of mesenchymal stem cells in the treatment of nonhealing wounds [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1081-1087. |
| [13] | Wang Shiqi, Zhang Jinsheng. Effects of Chinese medicine on proliferation, differentiation and aging of bone marrow mesenchymal stem cells regulating ischemia-hypoxia microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1129-1134. |
| [14] | Kong Desheng, He Jingjing, Feng Baofeng, Guo Ruiyun, Asiamah Ernest Amponsah, Lü Fei, Zhang Shuhan, Zhang Xiaolin, Ma Jun, Cui Huixian. Efficacy of mesenchymal stem cells in the spinal cord injury of large animal models: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1142-1148. |
| [15] | Chen Junyi, Wang Ning, Peng Chengfei, Zhu Lunjing, Duan Jiangtao, Wang Ye, Bei Chaoyong. Decalcified bone matrix and lentivirus-mediated silencing of P75 neurotrophin receptor transfected bone marrow mesenchymal stem cells to construct tissue-engineered bone [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 510-515. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||