Chinese Journal of Tissue Engineering Research ›› 2019, Vol. 23 ›› Issue (9): 1434-1440.doi: 10.3969/j.issn.2095-4344.1590
Previous Articles Next Articles
Biological characteristics of stromal cell-derived factor-1/CXC chemokine receptor 4 signal axis
Wu Zhouling1, 2, Basheer Hamed Hamood AL-Shameri1, 2, Ban Guifei2, Chen Wenxia2
-
Revised:2018-11-30Online:2019-03-28Published:2019-03-28 -
Contact:Chen Wenxia, PhD, Professor, Department of Operative Dentistry and Endodontology, Affiliated Stomatological Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China -
About author:Wu Zhouling, Master candidate, Guangxi Key Laboratory of Oral and Maxillofacial Rehabilitation and Reconstruction, Guangxi Clinical Research Center for Craniofacial Deformity, Guangxi Key Laboratory of Oral and Maxillofacial Surgery Disease Treatment, School of Stomatology, Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China; Department of Operative Dentistry and Endodontology, Affiliated Stomatological Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China -
Supported by:the National Natural Science Foundation of China, No. 81560183 (to CWX)
CLC Number:
Cite this article
Wu Zhouling, Basheer Hamed Hamood AL-Shameri, Ban Guifei, Chen Wenxia. Biological characteristics of stromal cell-derived factor-1/CXC chemokine receptor 4 signal axis[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(9): 1434-1440.
share this article
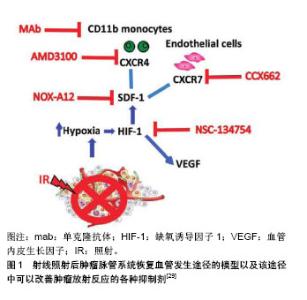
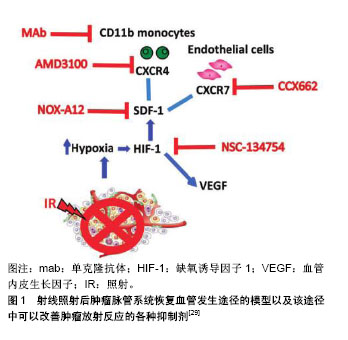
2.1 SDF-1和CXCR4的基本概念 SDF-1,也称趋化因子配体12(Chemokine(C-X-C motif) ligand 12,CXCL-12),是CXC趋化因子家族的成员之一。其最早是在小鼠骨髓中的基质细胞分泌的细胞因子中发现,它是一类能够使多种细胞发生趋化运动的细胞因子,因其参与前B细胞的增殖分化并在这一过程中起重要作用,所以又称为前B细胞刺激因子。SDF-1有很多亚型,包括有α,β,γ,δ,ε和ψ,其中SDF-1α在多种组织中广泛表达,并且调节黏附、趋化和存活等多种生命活动过程[5]。 CXCR4是一种具有7次跨膜结构的G蛋白耦联受体,由352个氨基酸残基构成,能表达于多种细胞表面,包括CD34+造血干/祖细胞、单核/巨噬细胞、胸腺细胞、前B细胞、树突状细胞、外周血淋巴细胞和血小板等[6-8],在内皮细胞、内皮祖细胞表面也可见其高表达[9]。SDF-1的受体有CXCR4和CXCR7,SDF-1与CXCR4的结合是研究较多的一种结合方式,SDF-1与受体CXCR4结合是其发挥生物学作用的前提。 SDF-1/CXCR4是配体受体复合物,但SDF-1并非CXCR4的惟一配体,有研究发现,巨噬细胞移动抑制因子、细胞外泛素、人类免疫缺陷病毒也能与CXCR4相互结合[10]。SDF-1与CXCR4结合后,形成具有强大生物学作用的信号轴,该信号轴在胚胎发育过程、机体的免疫及炎症反应、恶性肿瘤的浸润转移以及干细胞的迁徙和归巢等方面发挥作用[11]。SDF-1能与表达于造血祖细胞、内皮祖细胞、平滑肌祖细胞和成纤维祖细胞等多种祖细胞上的趋化因子受体CXCR4结合,继而发挥SDF-1/CXCR4信号轴的作用。 2.2 SDF-1和CXCR4的调节及相关研究 2.2.1 SDF-1的调节 SDF-1/CXCR4轴与细胞的迁移有密切的联系,高表达CXCR4的细胞能够向高浓度的SDF-1区域迁移是信号轴作用的结果[12]。在心肌梗死等急性缺血事件发生时,机体会动员骨髓中的干细胞释放进入血液,与此同时,梗死区SDF-1的浓度会显著的升高并在短时间内达到最大值,在心肌梗死区域高表达的SDF-1与外周血低表达的SDF-1之间形成SDF-1浓度梯度,从而趋化循环中的CXCR4阳性祖细胞归巢到缺血梗死部位[13]。SDF-1能趋化多种表达CXCR4干细胞归巢到心肌梗死区参与修复,这些干细胞包括间充质干细胞、造血干细胞、内皮祖细胞等。 心肌梗死后,炎症环境[14-16]、损伤组织、缺血缺氧因素都可上调SDF-1的表达[17-18],其中缺氧诱导因子1(hypoxia-inducible factor-1,HIF-1)是重要的调节因素。研究发现,SDF-1的启动子中包含有2个缺氧诱导因子1的接合位点,SDF-1的转录得以激活是缺氧诱导因子1连接位点后发挥作用的结果,转录后的SDF-1表达迅速升高,最终形成损伤区域高水平SDF-1、外周低水平SDF-1的浓度梯度[19]。此外,四肢缺血、肝脏药物损害、放疗及化疗药物损害等也会引起SDF-1表达增加[20],从而介导多种干细胞趋化性迁移,以修复受损的靶组织或器官。Ceradini等[21]的研究表明,在组织缺血、缺氧的情况下,缺氧诱导因子1能够快速的表达,在DNA损伤诱导剂和化学治疗的作用下,SDF-1的生成增多,在转录水平上,缺氧诱导因子1能够促进SDF-1表达上调,SDF-1的表达量与缺氧程度呈明显的正相关。 2.2.2 CXCR4的调节 众所周知,CXCR4的表达水平与SDF-1的功能密切相关,细胞表面CXCR4表达水平及CXCR4+细胞的比例较高时,细胞对SDF-1的反应性也明显升高。有研究发现,在进行骨髓间充质干细胞的体外培养时,培养时间延长以及细胞代数增加可导致骨髓间充质干细胞表面的CXCR4表达减少,使得SDF-1/CXCR4信号轴的作用减弱[22]。 采用病毒质粒转染CXCR4基因可以增强骨髓间充质干细胞对SDF-1的敏感性,但最近的研究发现,通过超声微气泡联合脂质体包被转染CXCR4到骨髓间充质干细胞,与传统的病毒质粒转染相比,该方法具有更高的转染效率,且Transwell实验表明该方法转染的骨髓间充质干细胞体外迁移能力与对照组相比提高了9倍[23]。既往已知阿托伐他汀能够促进SDF-1的分泌,且Li等[24]的研究证实,在体外用阿托伐他汀预处理间充质干细胞后可以提高间充质干细胞表面CXCR4的表达,有效的增加了大鼠尾静脉注射间充质干细胞后趋化到心肌梗死区域周围间充质干细胞的数量。此外,与组织损伤、应激有关的因子也能够正向调控CXCR4的表达,这些因子包括有核转录因子κB、缺氧诱导因子1、转化生长因子β及血管内皮生长因子等[21]。心肌梗死区域能够释放缺氧诱导因子1,而缺氧诱导因子1能提高骨髓间充质干细胞表面CXCR4受体的表达,增加梗死周边区细胞的驻留。Najafi等[25]发现采用铁螯合剂去铁胺预处理骨髓间充质干细胞能够上调CXCR4的表达,原因是去铁胺增加了缺氧诱导因子1的稳定性,从而间接使CXCR4表达增加。此外,胰高血糖素样肽1不仅可以稳定血糖,其受体激动剂exendin-4还能够通过PI3K/AKT途径上调脂肪来源间充质干细胞CXCR4的表达[26],从而增强脂肪干细胞的归巢能力。除了阿托伐他汀、去铁胺、exendin-4等上述因子或药物,既往还报道了粒细胞集落刺激因子[27]、红细胞生成素等因子也能够影响SDF-1/ CXCR4通路[28],正向调控间充质干细胞的归巢过程。 2.3 阻断SDF-1从而抑制SDF-1/CXCR4信号轴的实验研究 Olaptesed pegol(OLA-PEG,简称为NOX-A12)是一种新型聚乙二醇化(PEG)的镜像寡核苷酸(即所谓的镜像异构体,Spiegelmer®),由德国NOXXON制药公司生产,其与SDF-1具有高度亲和力而抑制SDF-1与受体CXCR4和CXCR7的结合,从而影响SDF-1的信号传导[29]。NOX-A12由45个L-对映体RNA核苷酸组成并在其5'末端携带相对分子质量40 000的聚乙二醇修饰以延长其血浆半衰期。寡核苷酸的45个L-对映体RNA核苷酸形成结构支架,其能以高亲和力识别并结合SDF-1,因此能有效阻断趋化因子SDF-1与CXCR4和CXCR7的相互作用[29]。 SDF-1与CXCR4结合后形成的SDF-1/CXCR4信号轴在介导炎症反应、细胞归巢、肿瘤转移、血管生成和组织再生等生理病理过程中发挥多重作用。研究结果显示NOX-A12能够抑制SDF-1对慢性淋巴细胞白血病患者体内癌细胞的趋化作用[30],当NOX-A12与硼替佐米联合使用时,可以降低小鼠多发性骨髓瘤中的肿瘤负荷[31]。在大鼠模型中NOX-A12显示出对抗胶质母细胞瘤活性的作用[29],在胶质母细胞瘤的治疗中还能逆转巨噬细胞的募集,与单独的抗血管内皮生长因子治疗相比,NOX-A12+抗血管内皮生长因子的组合显著降低了肿瘤相关巨噬细胞水平,显示出其具有增强抗血管内皮生长因子治疗方法治疗胶质母细胞瘤的效果[32]。另一项研究结果显示健康者对NOX-A12具有良好的耐受性,在白血病患者中表现出NOX-A12能剂量依赖性地将白细胞和造血干细胞动员到外周血中[33]。到目前为止,NOX-A12联合苯达莫司汀和利妥昔单抗用于治疗复发/难治性慢性淋巴细胞白血病,以及NOX-A12联合硼替佐米和地塞米松用于治疗复发/难治性多发性骨髓瘤患者的临床研究取得了令人鼓舞的结果[34]。前期研究发现,由于SDF-1表现为白血病细胞的存活因子,因此阻断SDF-1可能降低白血病癌细胞的活力[34]。有体外研究表明NOX-A12能够阻断SDF-1诱导的BCR-ABL阳性白血病细胞以及FLT3-ITD阳性白血病细胞的迁移能力[35],NOX-A12与酪氨酸激酶抑制剂联合使用能够显著的减少或根除单独使用酪氨酸激酶抑制剂后残留在骨髓微环境中的白血病相关CD34+祖细胞,其作用机制为NOX-A12在抑制SDF-1的同时,调节了肿瘤微环境,将白血病细胞动员到外周血,使得它们在外周血中更容易受到抗癌剂的细胞毒性作用。现阶段,NOX-A12正在进行Ⅱa期临床测试试验[36],以期其在临床应用中进行疾病治疗时能够更安全有效。肿瘤血管发生的机制以及在这一过程中可以使用的各种抑制剂见图1。"
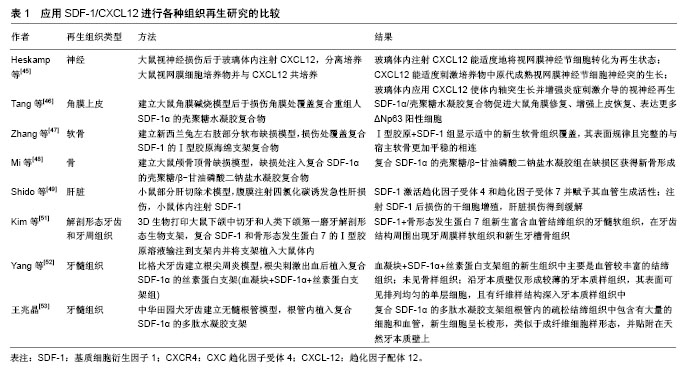
2.4 提高SDF-1增强SDF-1/CXCR4信号轴在组织再生中的研究 SDF-1可由各种组织细胞产生,包括神经、皮肤、肌肉、心脏、成骨细胞和血管周细胞等。SDF-1在多种干/祖细胞的招募聚集、迁徙和分化中发挥重要作用[41],能促进组织中血管的生成[42]、神经发生和骨生成[43-44]。目前为止,SDF-1已经应用于组织工程中并且进行了神经[45]、角膜上皮[46]、软骨[47]、骨以及肝脏等组织/器官的再生[48-49]。在小鼠后肢缺血模型中,SDF-1也有促进血液流动、血管新生和肌肉再生的作用[50]。 近年来,应用SDF-1进行牙髓再生方面的研究也取得了一定的成果。Kim等[51]证明,将复合SDF-1,骨形态发生蛋白7和Ⅰ型胶原溶液注射到与人类下颌第一磨牙解剖结构类似形状的3D生物支架内,可以形成富含血管结缔组织的牙髓软组织,并且在牙齿结构周围出现牙周膜样软组织和新生牙槽骨组织,复合SDF-1和骨形态发生蛋白7的实验组相对于无任何生长因子的对照组具有更多的新生血管(P < 0.05)。Yang等[52]在无细胞移植的情况下进行比格犬的牙髓血运重建并在根管内植入搭载有SDF-1的丝素蛋白支架以研究牙髓再生过程中伴随着的自噬。实验选择2只比格犬根尖完全闭合的成熟前磨牙为实验牙,并诱导根尖周病变。根管经机械预备和化学预备消毒后,刺激根尖部出血并形成血凝块,将搭载有SDF-1的丝素蛋白支架植入根管的血凝块中,对照组只进行牙髓血运重建术。术后3个月,对标本进行组织学分析。血凝块对照组再生组织中主要为沿牙本质壁形成的较厚的牙骨质样组织,髓腔内有大小不一的骨样组织及少量含有小血管的结缔组织。搭载有SDF-1的支架组再生组织为牙髓样组织,表现为血运较丰富的结缔组织,胶原纤维排列方式与正常牙髓类似,髓腔内未见骨样组织,沿牙本质壁形成较薄旳矿化组织。牙髓样组织的形成可能归因于SDF-1在新血管形成和矿化中的作用以及其趋化功能。实验同时通过免疫染色检查再生组织中自噬的存在:Atg5和LC3(自噬相关蛋白)在两组中均呈阳性,但SDF-1组阳性细胞数远高于对照组,因此可以得出,SDF-1引发了自噬的激活。该研究表明,搭载SDF-1的丝素蛋白支架可有效促进犬成熟牙齿无髓根管内牙髓血运重建的牙髓再生,从而建立了SDF-1在临床上进行牙髓再生可能的模型。除此之外,王兆晶[53]也对SDF-1在牙髓再生中的生物学特性进行了实验探索研究。实验建立犬成熟牙齿的无髓根管模型,无髓根管内植入复合SDF-1的多肽水凝胶支架作为实验组,单纯植入多肽水凝胶支架作为对照组。术后6周对标本进行组织学分析发现,实验组根管内的疏松结缔组织中包含有大量的细胞和血管,新生细胞呈长梭形,类似于成纤维细胞样形态,并贴附在天然牙本质壁上,而对照组根管内则主要为均质疏松的物质,无细胞、血管等组织成分和结构。该实验表明,无髓根管内植入复合SDF-1的多肽水凝胶胶原支架可诱导机体内源性细胞发生归巢并进行分化,使得无髓根管内再生牙髓样组织。应用SDF-1进行各种组织再生的实验研究见表1。"
| [1] Raedel M, Hartmann A, Bohm S, et al. Three-year outcomes of root canal treatment: Mining an insurance database. J Dent. 2015;43(4):412-417. [2] Burry JC, Stover S, Eichmiller F, et al. Outcomes of primary endodontic therapy provided by endodontic specialists compared with other providers. J Endod. 2016;42(5):702-705. [3] 李超然,黄桂林,王帅.间充质干细胞来源外泌体促进损伤组织修复与再生的应用与进展[J].中国组织工程研究,2018,22(1):133-139.[4] 陈慧鸿,韦颂观,庞博,等.牙根盾技术在保存前牙区种植体周围软硬组织的研究进展[J].中国组织工程研究,2018,22(10):1605-1610.[5] Ji Y. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107(9):1322-1328. [6] Loetscher M, Geiser T, O'Reilly T, et al. Cloning of a human seven-transmembrane domain receptor, LESTR, that is highly expressed in leukocytes. J Biol Chem. 1994;269(1):232-237. [7] Wang JF, Liu ZY, Groopman JE. The α-chemokine receptor CXCR4 is expressed on the megakaryocytic lineage from progenitor to platelets and modulates migration and adhesion. Blood. 1998;92(3):756-764. [8] Aiuti A, Tavian M, Cipponi A, et al. Expression of CXCR4, the receptor for stromal cell-derived factor-1 on fetal and adult human lymphohematopoietic progenitors. Eur J Immunol. 1999;29: 1823-1831. [9] Stellos K, Langer H, Daub K, et al. Platelet-derived stromal cell-derived factor-1 regulates adhesion and promotes differentiation of human CD34+ cells to endothelial progenitor cells. Circulation. 2008;117(2):206-215. [10] Pawig L, Klasen C, Weber C, et al. Diversity and inter-connections in the CXCR4 chemokine receptor/ligand family: molecular perspectives. Front Immunol. 2015;6(429):1-23. [11] Anders HJ, Romagnani P, Mantovani A. Pathomechanisms: homeostatic chemokines in health, tissue regeneration, and progressive diseases. Trends Mol Med. 2014; 20(3):154-165. [12] Arimitsu N, Shimizu J, Fujiwara N, et al. Role of SDF1/CXCR4 interaction in experimental hemiplegic models with neural cell transplantation. Int J Mol Sci. 2012;13(3):2636-2649. [13] Cheng M, Huang K, Zhou J, et al. A critical role of Src family kinase in SDF-1/CXCR4-mediated bone-marrow progenitor cell recruitment to the ischemic heart. J Mol Cell Cardiol. 2015;81: 49-53. [14] Gong J, Meng HB, Hua J, et al. The SDF-1/CXCR4 axis regulates migration of transplanted bone marrow mesenchymal stem cells towards the pancreas in rats with acute pancreatitis. Mol Med Rep. 2014;9(5):1575-1582. [15] Shi H, Lu R, Wang S, et al. Effects of SDF-1/CXCR4 on acute lung injury induced by cardiopulmonary bypass. Inflammation. 2017;40(3):937-945. [16] Doring Y, Pawig L, Weber C, et al. The CXCL12/CXCR4 chemokine ligand/receptor axis in cardiovascular disease. Front Physiol. 2014;5(212):1-23. [17] Cotoia A, Mirabella L, Altamura S, et al. Circulating stem cells, HIF-1, and SDF-1 in septic abdominal surgical patients: randomized controlled study protocol. Trials. 2018;19(179):1-5. [18] McClendon J, Jansing NL, Redente EF, et al. Hypoxia-inducible factor 1α signaling promotes repair of the alveolar epithelium after acute lung injury. Am J Pathol. 2017;187(8):1772-1786. [19] Choi JH, Nguyen MP, Lee D, et al. Hypoxia-induced endothelial progenitor cell function is blunted in angiotensinogen knockout mice. Mol Cells. 2014;37(6):487-496. [20] Kucia M, Reca R, Miekus K, et al. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23(7): 879-894. [21] Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10(8):858-864. [22] Wynn RF, Hart CA, Corradi-Perini C, et al. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104(9):2643-2645. [23] Wang G, Zhuo Z, Zhang Q, et al. Transfection of CXCR-4 using microbubble-mediated ultrasound irradiation and liposomes improves the migratory ability of bone marrow stromal cells. Curr Gene Ther. 2015;15(1):21-31. [24] Li N, Yang YJ, Qian HY, et al. Intravenous administration of atorvastatin-pretreated mesenchymal stem cells improves cardiac performance after acute myocardial infarction: role of CXCR4. Am J Transl Res. 2015;7(6):1058-1070. [25] Najafi R1, Sharifi AM. Deferoxamine preconditioning potentiates mesenchymal stem cell homing in vitro and in streptozotocin- diabetic rats. Expert Opin Biol Ther. 2013;13(7): 959-972. [26] Zhou H, Yang J, Xin T, et al. Exendin-4 enhances the migration of adipose-derived stem cells to neonatal rat ventricular cardiomyocyte- derived conditioned medium via the phosphoinositide 3-kinase/ Akt-stromal cell-derived factor-1alpha/ CXC chemokine receptor 4 pathway. Mol Med Rep. 2015;11(6): 4063-4072. [27] Mendt M, Cardier JE. Role of SDF-1 (CXCL12) in regulating hematopoietic stem and progenitor cells traffic into the liver during extramedullary hematopoiesis induced by G-CSF, AMD3100 and PHZ. Cytokine. 2015;76(2):214-221. [28] Li J, Guo W, Xiong M, et al. Effect of SDF-1/CXCR4 axis on the migration of transplanted bone mesenchymal stem cells mobilized by erythropoietin toward lesion sites following spinal cord injury. Int J Mol Med. 2015;36(5):1205-1214. [29] Liu SC, Alomran R, Chernikova SB, et al. Blockade of SDF-1 after irradiation inhibits tumor recurrences of autochthonous brain tumors in rats. Neuro Oncol. 2014;16(1):21-28. [30] Hoellenriegel J, Zboralski D, Maasch C, et al. The spiegelmer NOX-A12, a novel CXCL12 inhibitor, interferes with chronic lymphocytic leukemia cell motility and causes chemosensitization. Blood. 2014;123(7):1032-1039. [31] Roccaro AM, Sacco A, Purschke WG, et al. SDF-1 inhibition targets the bone marrow niche for cancer therapy. Cell Rep. 2014; 9(1):118-128. [32] Deng L, Stafford JH, Liu SC, et al. SDF-1 blockade enhances anti-VEGF therapy of glioblastoma and can be monitored by MRI. Neoplasia. 2017;19(1):1-7. [33] Vater A, Sahlmann J, Kroger N, et al. Hematopoietic stem and progenitor cell mobilization in mice and humans by a first-in-class mirror-image oligonucleotide inhibitor of CXCL12. Clin Pharmacol Ther. 2013;94(1):150-157. [34] Vater A, Klussmann S. Turning mirror-image oligonucleotides into drugs: the evolution of Spiegelmer® therapeutics. Drug Discov Today. 2015;20(1):147-155. [35] Weisberg EL, Sattler M, Azab AK, et al. Inhibition of SDF-1-induced migration of oncogene-driven myeloid leukemia by the L-RNA aptamer (Spiegelmer), NOX-A12, and potentiation of tyrosine kinase inhibition. Oncotarget. 2017;8(66): 109973-109984. [36] Ludwig H, Weisel K, Petrucci MT, et al. Olaptesed pegol, an anti-CXCL12/SDF-1 Spiegelmer, alone and with bortezomib- dexamethasone in relapsed/refractory multiple myeloma: a phase Ⅱa study. Leukemia. 2017;31(4):997-1000. [37] Li XQ, Zhang ZL, Tan WF, et al. Down-regulation of CXCL12/ CXCR4 expression alleviates ischemia-reperfusion- induced inflammatory pain via inhibiting glial TLR 4 activation in the spinal cord. PLoS One. 2016;11(10):1-14. [38] Isaacson B, Hadad T, Glasner A, et al. Stromal cell-derived factor 1 mediates immune cell attraction upon urinary tract infection. Cell Rep. 2017;20(1):40-47. [39] Liu JY, Chen X, Yue L, et al. CXC chemokine receptor 4 is expressed paravascularly in apical papilla and coordinates with stromal cell-derived factor-1alpha during transmigration of stem cells from apical papilla. J Endod. 2015;41(9):1430-1436. [40] Chen X, Liu JY, Yue L, et al. Phosphatidylinositol 3-kinase and protein kinase c signaling pathways are involved in stromal cell–derived factor-1α–mediated transmigration of stem cells from apical papilla. J Endod. 2016;42(7):1076-1081. [41] Liekens S, Schols D, Hatse S. Hatse. CXCL12-CXCR4 axis in angiogenesis, metastasis and stem cell mobilization. Curr Pharmaceutical Design. 2010;16(35):3903-3920. [42] Perrucci GL, Straino S, Corliano M, et al. Cyclophilin A modulates bone marrow-derived CD117(+) cells and enhances ischemia-induced angiogenesis via the SDF-1/CXCR4 axis. Int J Cardiol. 2016;212:324-335. [43] Cheng X, Wang H, Zhang X, et al. The role of SDF-1/CXCR4/ CXCR7 in neuronal regeneration after cerebral ischemia. Front Neurosci. 2017;11(590):1-13. [44] Hwang HD, Lee JT, Koh JT, et al. Sequential treatment with SDF-1 and BMP-2 potentiates bone formation in calvarial defects. Tissue Eng Part A. 2015;21(13-14):2125-2135. [45] Heskamp A, Leibinger M, Andreadaki A, et al. CXCL12/SDF-1 facilitates optic nerve regeneration. Neurobiol Dis. 2013;55:76-86. [46] Tang Q, Luo C, Lu B, et al. Thermosensitive chitosan-based hydrogels releasing stromal cell derived factor-1 alpha recruit MSC for corneal epithelium regeneration. Acta Biomater. 2017;61: 101-113. [47] Zhang W, Chen J, Tao J, et al. The use of type 1 collagen scaffold containing stromal cell-derived factor-1 to create a matrix environment conducive to partial-thickness cartilage defects repair. Biomaterials. 2013;34(3):713-723. [48] Mi L, Liu H, Gao Y, et al. Injectable nanoparticles/hydrogels composite as sustained release system with stromal cell-derived factor-1 alpha for calvarial bone regeneration. Int J Biol Macromol. 2017;101:341-347. [49] Shido K, Chavez D, Cao Z, et al. Platelets prime hematopoietic and vascular niche to drive angiocrine-mediated liver regeneration. Signal Transduct Target Ther. 2017. doi: 10.1038/ sigtrans.2016.44. [50] Kuliszewski MA, Kobulnik J, Lindner JR, et al. Vascular gene transfer of SDF-1 promotes endothelial progenitor cell engraftment and enhances angiogenesis in ischemic muscle. Mol Ther. 2011;19(5):895-902. [51] Kim K, Lee CH, Kim BK, et al. Anatomically shaped tooth and periodontal regeneration by cell homing. J Dent Res. 2010;89(8): 842-847. [52] Yang JW, Zhang YF, Wan CY, et al. Autophagy in SDF-1alpha- mediated DPSC migration and pulp regeneration. Biomaterials. 2015;44:11-23. [53] 王兆晶.基质衍生细胞因子-1α诱导内源性细胞归巢进行牙髓再生的硏究[D].南宁:广西医科大学,2016.[54] Fawzy El-Sayed KM, Jakusz K, Jochens A, et al. Stem cell transplantation for pulpal regeneration: a systematic review. Tissue Eng Part B Rev. 2015;21(5):451-460. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [3] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [4] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [5] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [6] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [7] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [8] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [9] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [10] | Li Jun, Zuo Xinhui, Liu Xiaoyuan, Zhang Kai, Han Xiangzhen, He Huiyu, . Effect of over expression of miR-378a on osteogenic and vascular differentiation of bone marrow mesenchymal stem cell sheet [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(31): 4939-4944. |
| [11] | Li Junqing, He Wenxi, Guo Qian, Wu Jiayuan. Effect of hydrostatic pressure on odontogenic/osteogenic differentiation of dental pulp stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(31): 4976-4980. |
| [12] | Xing Hao, Zhang Yonghong, Wang Dong. Advantages and disadvantages of repairing large-segment bone defect [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 426-430. |
| [13] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| [14] | Wang Hao, Chen Mingxue, Li Junkang, Luo Xujiang, Peng Liqing, Li Huo, Huang Bo, Tian Guangzhao, Liu Shuyun, Sui Xiang, Huang Jingxiang, Guo Quanyi, Lu Xiaobo. Decellularized porcine skin matrix for tissue-engineered meniscus scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3473-3478. |
| [15] | Mo Jianling, He Shaoru, Feng Bowen, Jian Minqiao, Zhang Xiaohui, Liu Caisheng, Liang Yijing, Liu Yumei, Chen Liang, Zhou Haiyu, Liu Yanhui. Forming prevascularized cell sheets and the expression of angiogenesis-related factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3479-3486. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||