Chinese Journal of Tissue Engineering Research ›› 2018, Vol. 22 ›› Issue (29): 4729-4735.doi: 10.3969/j.issn.2095-4344.0633
Previous Articles Next Articles
Adipose-derived stem cells for treating knee osteoarthritis: relevant clinical trials and registration
Pang Zhi-ying1, Yin Feng2, Yuan Feng2
- 1Shanghai Medical College of Fudan University, Shanghai 200032, China; 2Department of Joint and Joint Diseases, Shanghai Dongfang Hospital, Shanghai 200120, China
-
Revised:2018-05-15Online:2018-10-18Published:2018-10-18 -
Contact:Yuan Feng, MD, Associate chief physician, Department of Joint and Joint Diseases, Shanghai Dongfang Hospital, Shanghai 200120, China -
About author:Pang Zhi-ying, Doctorate candidate, Physician, Shanghai Medical College of Fudan University, Shanghai 200032, China -
Supported by:the Funded Project of Shanghai Municipal Science and Technology Committee, No. 15411968700; the Special Department Construction of the Health System of Pudong New Area, Shanghai, No. PWZzk2017-25
CLC Number:
Cite this article
Pang Zhi-ying, Yin Feng, Yuan Feng. Adipose-derived stem cells for treating knee osteoarthritis: relevant clinical trials and registration[J]. Chinese Journal of Tissue Engineering Research, 2018, 22(29): 4729-4735.
share this article
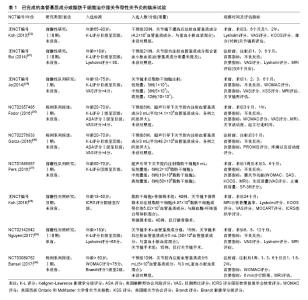
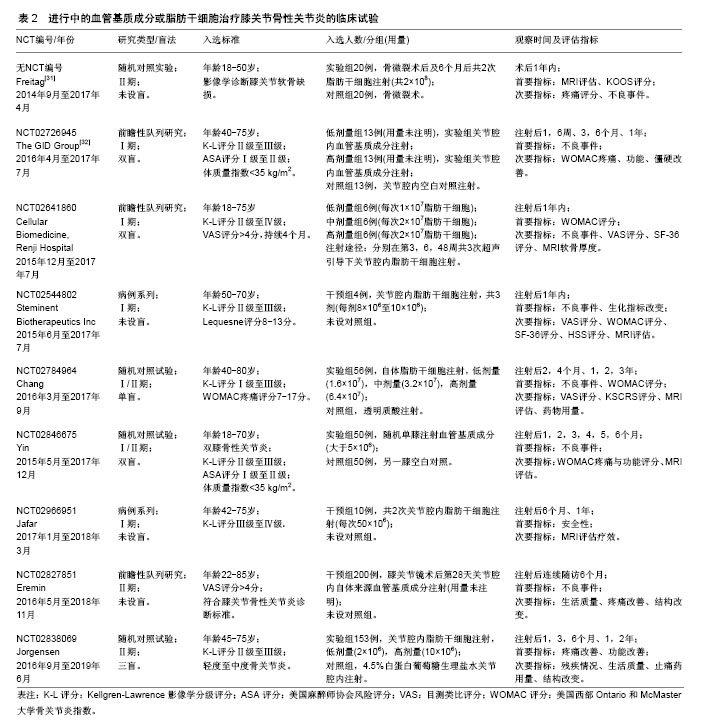
2.1 已完成临床试验数据分析 共有9篇已完成的临床试验文献报道,现将其试验设计列举见表1。Koh等[22]在韩国开展的临床试验中,入选标准为年龄 > 65岁,患者关节软骨损伤K-L评分Ⅱ级至Ⅲ级,非手术治疗3个月以上无效。总计入选30例患者,治疗方案是受试者在关节镜下灌洗后于受损最严重部位注射混合于3 mL富血小板血浆的4.2×107血管基质成分。随访2年后结果显示,受试者Lyshome评分(术前54.3±15.4,术后74.2±13.4)、目测类比(VAS)评分(术前4.7±1.6,术后1.7±1.4)、KOOS评分明显改善,部分患者(16例)术后2年进行了第2次关节镜手术,大部分受试者对血管基质成分注射的治疗效果呈“阳性”。证据等级为Ⅳ级。Bui等[23]在越南开展的临床试验中,入选标准为>18岁,患者关节软骨损伤K-L评分Ⅱ级至Ⅲ级,Lysholm评分<65分,药物或自体软骨移植治疗无效。总计入选21例患者,未设盲,治疗方案是注射血管基质成分+富血小板血浆,具体剂量未详述。随访6个月后结果显示,受试者在VAS评分(注射前7.6±0.5,注射后1.5±0.5)和Lysholm评分(注射前61±11,注射后82±8.1)方面有显著改善,MRI提示软骨厚度增厚,具体未详述。证据等级为Ⅳ级。 Jo等[24]在韩国开展的临床试验中,入选标准为18-75岁,患者关节软骨损伤K-L评分Ⅱ级至Ⅳ级,止痛药物治疗无效,VAS评分>4分,软骨损伤面积在2-6 cm2内。总计入选了18例患者,未设盲,分4组,3例接受低剂量(1×107)脂肪干细胞,3例接受中剂量(5×107)脂肪干细胞,3例接受高剂量(10×107)脂肪干细胞,注射 28 d后评估安全性,之后9例接受高剂量(10×107)脂肪干细胞。治疗方案是关节镜术后将各组不同用量的脂肪干细胞重悬于3 mL生理盐水,关节腔内注射。随访6个月后结果显示,高剂量组受试者在WOMAC评分(注射前54.2±5.2,注射后32.8±6.3)、VAS评分(注射前79.6±2.2,注射后44.2±6.3)、KSS评分(注射前47.2±2.6,注射后71.0±4.4)方面有显著改善,MRI提示软骨缺损面积明显减小,软骨总量增加。在对同一批受试者随访2年结果显示[24],高剂量组受试者在WOMAC评分(注射前54.2±5.2,注射后19.0±5.5)、VAS评分(注射前79.6±2.2,注射后45.8±8.1)、KSS评分(注射前47.2±2.6,注射后79.3±4.7)方面有显著改善,改善程度较6个月随访差异更为明显。证据等级为Ⅱ级。 Fodor等[25]在美国开展的临床试验中,入选标准为20-70岁,患者关节软骨损伤K-L评分Ⅱ级至Ⅲ级,VAS评分>4分,既往治疗无效。总计入选6例患者,未设盲,治疗方案是受试者接受膝关节腔内14.1×106血管基质成分混合于3 mL乳酸林格溶液注射。随访1年后结果显示,受试者在WOMAC评分(注射前32.9 ±14.6,注射后9.4±10.1)、VAS评分(注射前5.9±1.2,注射后2.0±1.8)方面有明显改善,关节活动度[注射前(136.6±7.3)°,注射后3个月(143.6±6.7)°]略有改善,起立时间[注射前(5.4±1.6) s,注射后3个月(2.8±0.3) s]明显改善。证据等级为Ⅳ级。 Garza等[26]在美国开展的临床试验中,入选标准为20-70岁,患者关节软骨损伤K-L评分Ⅱ级至Ⅲ级,ASA评分Ⅰ级至Ⅱ级,体质量指数小于35 kg/m2,VAS评分>4。总计入选6例患者,未设盲,治疗方案是超声下关节腔内注射48.2×106血管基质成分。随访3个月后结果显示受试者PROMIS疼痛评分有明显改善。证据等级为Ⅳ级。 Pers等[27]在欧洲的临床试验中,入选标准为50-70岁,患者关节软骨损伤K-L评分Ⅲ级至Ⅳ级,持续12个月以上膝关节疼痛。总计入选18例患者,未设盲,低剂量组6例(2×106脂肪干细胞),中剂量组6例(10×106脂肪干细胞),高剂量组6例(50×106脂肪干细胞)。治疗方案是受试者接受超声下膝关节腔内注射混合于3.6%白蛋白的相应数量脂肪干细胞共5 mL。随访6个月后结果显示,各组受试者在WOMAC评分、SF-36评分等方面均显示出相比于注射前明显改善,影像学方面dGEMRIC和T1加权MRI未在受试者中得到一致性改变,11例受试者进行了组织学评分。证据等级为Ⅱ级。 Koh等[28]在韩国的随机对照试验中,入选标准为18-50岁,患者关节软骨损伤ICRS评分Ⅲ级至Ⅳ级。总计入选80例患者,骨微裂术组40例,骨微裂术+脂肪干细胞组40例,分组对受试者设盲。治疗方案是所有受试者接受关节镜手术,术中清除损伤软骨,在软骨下进行骨微裂术,术后骨微裂术+脂肪干细胞组注射4.97×106脂肪干细胞或等价的5.02×107血管基质成分1︰1混合于凝血酶-纤维蛋白原溶液注射在缺损软骨表面。随访2年后结果显示,受试者在MRI MOCART评分(骨微裂术+脂肪干细胞组:36.6±11.9,骨微裂术组:51.8±19.7)有明显改善,KOOS评分仅在部分量表(疼痛评分,骨微裂术+脂肪干细胞组:62.4±15.3,骨微裂术组:30.1± 14.7;症状评分,骨微裂术+脂肪干细胞组:32.3±7.2,骨微裂术组:27.8±6.8)有改善,Lyshome评分以及VAS评分明显改善,在57例进行第二次关节镜手术的受试者中,软骨损伤评级在两组间无显著差异,软骨损伤ICRS评分有明显改善。证据等级为Ⅱ级。 Nguyen等[29]在越南的随机对照试验中,入选标准为年龄>18岁,患者关节软骨损伤K-L评分Ⅱ级至Ⅲ级,Lysholm评分<65分,药物或自体软骨移植治疗无效。总计入选30例患者,血管基质成分组15例,对照组15例。治疗方案是所有受试者接受关节镜手术,术中清除损伤软骨,在软骨下进行骨微裂术,血管基质成分组注射混合于3 mL富血小板血浆的血管基质成分5 mL,对照组注射生理盐水。随访18个月后结果显示WOMAC评分、Lyshome评分以及VAS评分在两组间差异显著,MRI结果显示评分改变不一,在关节功能恢复方面明显改善。证据等级为Ⅱ级。 Bansal等[30]在印度开展的研究中,入选标准为年龄50岁以上,患者关节Brandt影像学评分Ⅰ级至Ⅱ级,疼痛持续3个月以上,WOMAC评分<75分。总计入选10例患者,未设盲,治疗方案是受试者接受5 mL重悬于生理盐水、与3 mL富血小板血浆混合的4.2×107血管基质成分关节腔内注射。随访2年后结果显示注射前后WOMAC评分明显改善,6 min步行距离改善,MRI显示软骨厚度改变不一。证据等级为Ⅳ级。 作者认为,截止目前已完成的脂肪干细胞治疗膝关节骨性关节炎的临床试验仅停留在Ⅰ期临床阶段。以上文献共计入选了219例受试者,各个临床试验的入选标准以及排除标准大相径庭。在年龄组的选择上有3个试验入选了高龄人群,其余均采用18岁以上常规年龄组。在提供年龄数据的198例受试者中,平均年龄53.2岁,女性占68.1%。入选标准中包含且不限于:影像学标准、基于临床诊断的临床标准以及基于疼痛或功能评价的量表标准。在试验设计上,未采用严格的脂肪干细胞-空白对照试验,部分试验仅使用单组设计,1个试验观察脂肪干细胞剂量和疗效关系,2个试验观察脂肪干细胞联合其他治疗对比该治疗的差异。在盲法设计上,大部分临床试验使用了开放性试验,即未对受试者设盲,仅有1个临床试验设单盲。试验的主要目的是观察整个治疗过程中不良事件的发生率,以上临床试验均未报道与治疗相关的严重不良反应事件,以此证明膝关节注射脂肪干细胞或血管基质成分的安全性。在其他评估疗效的观察指标中,仅设单组观察脂肪干细胞疗效的前瞻性研究显示,应用脂肪干细胞治疗前后VAS评分、WOMAC评分、Lysholm评分、MRI软骨评估、患者行动能力等方面有显著差异[22-23,25-26,30]。作者认为这些前瞻性研究的病例数量较小(6-30例),且存在混杂偏倚,即受试者在接受脂肪干细胞治疗前后的症状改善是由于各种其他混杂因素,如生活方式改善或安慰剂效应造成,因此这些未设对照的临床试验结果并无法准确评价脂肪干细胞治疗的有效性,其证据等级为Ⅳ级,等价于病例系列研究,对临床的指导意义有限。在设立对照组的临床试验结果中:2个剂量疗效试验中,1个结果显示对比注射前,在VAS评分、WOMAC评分等方面低剂量组(2×106脂肪干细胞)有着相较于其他剂量更显著的改善[27],而另1个结果显示仅在高剂量组(10×107脂肪干细胞)观察到WOMAC评分的显著改善[24]。以上矛盾的结果无法对今后临床试验脂肪干细胞用量作出指导,因此作者认为需要结合动物实验以及更大规模的剂量效应临床试验数据证明其确切的剂量疗效关系,以此指导脂肪干细胞治疗骨关节炎试验方案的制定。2个应用其他疗法对照脂肪干细胞联合该疗法的试验中,均显示治疗后对比治疗前在VAS评分、WOMAC评分、Lysholm评分、MRI软骨评估等方面有显著差异,然而显著的组间差异仅在一些特定观察点(例如仅在18个月观察到显著差异而不是6个月或12个月)或是一些量表的亚组(例如KOOS量表中的KOOS疼痛量表以及症状量表中观察到显著差异,而不是KOOS活动量表或生活质量量表或运动量表)[24]。以上实验结果的统计学差异可以部分说明脂肪干细胞的有效性,然而因为试验设计中存在偏倚,包括量表本身的偏倚、未设盲法导致受试者或实验者的主观偏倚,受试者数量的不足造成的偏倚以及采用回顾性数据作为对照组造成的选择性偏倚,因此这些对照试验的结论难以令人信服。作者认为这些研究属于低质量随机对照试验,其确切临床意义和研究价值有待商榷。综上所述,所纳入的临床研究并不能证明脂肪干细胞治疗骨关节炎的有效性。 2.2 进行中临床试验数据分析 共有9个临床试验正在进行,其试验设计列举见表2。 作者认为现在正在进行的应用脂肪干细胞或血管基质成分治疗膝关节骨性关节炎的临床试验属于Ⅱ期临床试验。以上文献拟入选570例受试者,试验的入选标准与目前已完成的临床试验相仿,而在入选人数、盲法、对照组设计等方面做到了进一步完善。随机对照试验拟入选人数达到了40-200例;盲法上基本均对受试者设盲,更有1个试验拟做到三盲;Yin和The GID Group的试验中空白组的设计均做到了真正意义的空白对照。试验的主要目的仍是观察不良事件,其次是观察脂肪干细胞或血管基质成分在改善疼痛、功能、生活质量方面进行量表评估;在软骨修复方面进行MRI评估。由于缺少具体文献佐证,作者无法对这些临床试验进行证据分级,但可以肯定的是这些随机对照试验结果将会为是否应用脂肪干细胞治疗膝关节骨性关节炎作出定论。"

| [1] Kang X, Fransen M, Zhang Y, et al. The high prevalence of knee osteoarthritis in a rural Chinese population: the Wuchuan osteoarthritis study. Arthritis Rheum. 2009;61(5):641-647.[2] Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1323-1330.[3] McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(3):363-388.[4] Insall JN, Ranawat CS, Aglietti P, et al. A comparison of four models of total knee-replacement prostheses. J Bone Joint Surg Am. 1976; 58(6):754-765.[5] Cram P, Lu X, Kates SL, et al. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991-2010. JAMA. 2012;308(12):1227-1236.[6] Gill GS, Joshi AB. Long-term results of cemented, posterior cruciate ligament-retaining total knee arthroplasty in osteoarthritis. Am J Knee Surg. 2001;14(4):209-214.[7] Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16(2):137-162.[8] Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21(9):571-576.[9] Wylde V, Hewlett S, Learmonth ID, et al. Persistent pain after joint replacement: prevalence, sensory qualities, and postoperative determinants. Pain. 2011;152(3):566-572.[10] Bayliss LE, Culliford D, Monk AP, et al. The effect of patient age at intervention on risk of implant revision after total replacement of the hip or knee: a population-based cohort study. Lancet. 2017;389 (10077):1424-1430.[11] Thorlund JB, Juhl CB, Roos EM, et al. Arthroscopic surgery for degenerative knee: systematic review and meta-analysis of benefits and harms. BMJ. 2015;350:h2747.[12] Rodbell M. Metabolism of isolated fat cells. II. The similar effects of phospholipase C (Clostridium perfringens alpha toxin) and of insulin on glucose and amino acid metabolism. J Biol Chem. 1966;241(1): 130-139.[13] Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211-228.[14] Bourin P, Bunnell BA, Casteilla L, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013;15(6):641-648.[15] Naderi N, Combellack EJ, Griffin M, et al. The regenerative role of adipose-derived stem cells (ADSC) in plastic and reconstructive surgery. Int Wound J. 2017;14(1):112-124.[16] Wang W, He N, Feng C, et al. Human adipose-derived mesenchymal progenitor cells engraft into rabbit articular cartilage. Int J Mol Sci. 2015;16(6):12076-12091.[17] Li M, Luo X, Lv X, et al. In vivo human adipose-derived mesenchymal stem cell tracking after intra-articular delivery in a rat osteoarthritis model. Stem Cell Res Ther. 2016;7(1):160.[18] Tang Y, Pan ZY, Zou Y, et al. A comparative assessment of adipose-derived stem cells from subcutaneous and visceral fat as a potential cell source for knee osteoarthritis treatment. J Cell Mol Med. 2017;21(9):2153-2162.[19] Pak J. Regeneration of human bones in hip osteonecrosis and human cartilage in knee osteoarthritis with autologous adipose-tissue-derived stem cells: a case series. J Med Case Rep. 2011;5:296.[20] Freitag J, Shah K, Wickham J, et al. The effect of autologous adipose derived mesenchymal stem cell therapy in the treatment of a large osteochondral defect of the knee following unsuccessful surgical intervention of osteochondritis dissecans - a case study. BMC Musculoskelet Disord. 2017;18(1):298.[21] Gibbs N, Diamond R, Sekyere EO, et al. Management of knee osteoarthritis by combined stromal vascular fraction cell therapy, platelet-rich plasma, and musculoskeletal exercises: a case series. J Pain Res. 2015;8:799-806.[22] Koh YG, Choi YJ, Kwon SK, et al. Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2015;23(5):1308-1316.[23] Bui HT, Duong TD, Nguyen NT, et al. Symptomatic knee osteoarthritis treatment using autologous adipose derived stem cells and platelet-rich plasma: A clinical study. Biomedical Research & Therapy. 2014; 1(1):2-8.[24] Jo CH, Lee YG, Shin WH, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;32(5): 1254-1266.[25] Fodor PB, Paulseth SG. Adipose Derived Stromal Cell (ADSC) Injections for Pain Management of Osteoarthritis in the Human Knee Joint. Aesthet Surg J. 2016;36(2):229-236.[26] Garza JR, Santa Maria D, Palomera T,et al. Use of Autologous Adipose-Derived Stromal Vascular Fraction to Treat Osteoarthritis of the Knee: A Feasibility and Safety Study. J Regen Med. 2015.[27] Pers YM, Rackwitz L, Ferreira R, et al. Adipose Mesenchymal Stromal Cell-Based Therapy for Severe Osteoarthritis of the Knee: A Phase I Dose-Escalation Trial. Stem Cells Transl Med. 2016;5(7): 847-856.[28] Koh YG, Kwon OR, Kim YS, et al. Adipose-Derived Mesenchymal Stem Cells With Microfracture Versus Microfracture Alone: 2-Year Follow-up of a Prospective Randomized Trial. Arthroscopy. 2016; 32(1):97-109.[29] Nguyen PD, Tran TD, Nguyen HT, et al. Comparative Clinical Observation of Arthroscopic Microfracture in the Presence and Absence of a Stromal Vascular Fraction Injection for Osteoarthritis. Stem Cells Transl Med. 2017;6(1):187-195.[30] Bansal H, Comella K, Leon J, et al. Intra-articular injection in the knee of adipose derived stromal cells (stromal vascular fraction) and platelet rich plasma for osteoarthritis. J Transl Med. 2017;15(1): 141.[31] Freitag J, Ford J, Bates D, et al. Adipose derived mesenchymal stem cell therapy in the treatment of isolated knee chondral lesions: design of a randomised controlled pilot study comparing arthroscopic microfracture versus arthroscopic microfracture combined with postoperative mesenchymal stem cell injections. BMJ Open. 2015;5(12):e009332.[32] Cimino WW, Llull R, Katz AJ. Preparation of compositions to treat and treatment of osteoarthritis using adipose-derived stromal vascular fraction cells[Z]. Google Patents, 2016.[33] Kirkley A, Birmingham TB, Litchfield RB, et al. A randomized trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2008;359(11):1097-1107.[34] Skou ST, Roos EM, Laursen MB, et al. A Randomized, Controlled Trial of Total Knee Replacement. N Engl J Med. 2015;373(17): 1597-1606.[35] Pak J, Lee JH, Kartolo WA, et al. Cartilage Regeneration in Human with Adipose Tissue-Derived Stem Cells: Current Status in Clinical Implications. Biomed Res Int. 2016;2016:4702674. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [3] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [4] | Peng Zhihao, Feng Zongquan, Zou Yonggen, Niu Guoqing, Wu Feng. Relationship of lower limb force line and the progression of lateral compartment arthritis after unicompartmental knee arthroplasty with mobile bearing [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1368-1374. |
| [5] | Huang Dengcheng, Wang Zhike, Cao Xuewei. Comparison of the short-term efficacy of extracorporeal shock wave therapy for middle-aged and elderly knee osteoarthritis: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1471-1476. |
| [6] | Liu Xiangxiang, Huang Yunmei, Chen Wenlie, Lin Ruhui, Lu Xiaodong, Li Zuanfang, Xu Yaye, Huang Meiya, Li Xihai. Ultrastructural changes of the white zone cells of the meniscus in a rat model of early osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1237-1242. |
| [7] | Zhang Xiumei, Zhai Yunkai, Zhao Jie, Zhao Meng. Research hotspots of organoid models in recent 10 years: a search in domestic and foreign databases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1249-1255. |
| [8] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [9] | Shi Yangyang, Qin Yingfei, Wu Fuling, He Xiao, Zhang Xuejing. Pretreatment of placental mesenchymal stem cells to prevent bronchiolitis in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 991-995. |
| [10] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [11] | Fan Quanbao, Luo Huina, Wang Bingyun, Chen Shengfeng, Cui Lianxu, Jiang Wenkang, Zhao Mingming, Wang Jingjing, Luo Dongzhang, Chen Zhisheng, Bai Yinshan, Liu Canying, Zhang Hui. Biological characteristics of canine adipose-derived mesenchymal stem cells cultured in hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1002-1007. |
| [12] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [13] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [14] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| [15] | Duan Liyun, Cao Xiaocang. Human placenta mesenchymal stem cells-derived extracellular vesicles regulate collagen deposition in intestinal mucosa of mice with colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1026-1031. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||