Chinese Journal of Tissue Engineering Research ›› 2018, Vol. 22 ›› Issue (29): 4721-4728.doi: 10.3969/j.issn.2095-4344.0632
Previous Articles Next Articles
Premature senescence of mesenchymal stem cells: causes and preventive measures
Zhou Long1, He Fan2, Wang Lei1
- 1Department of Orthopaedics, the Affiliated Suzhou Hospital of Nanjing Medical University (Suzhou Science and Technology Town Hospital), Suzhou 215153, Jiangsu Province, China; 2Orthopaedic Institute, Soochow University, Suzhou 215007, Jiangsu Province, China
-
Revised:2018-07-16Online:2018-10-18Published:2018-10-18 -
Contact:Wang Lei, Associate chief physician, Department of Orthopaedics, the Affiliated Suzhou Hospital of Nanjing Medical University (Suzhou Science and Technology Town Hospital), Suzhou 215153, Jiangsu Province, China -
About author:Zhou Long, Master, Physician, Department of Orthopaedics, the Affiliated Suzhou Hospital of Nanjing Medical University (Suzhou Science and Technology Town Hospital), Suzhou 215153, Jiangsu Province, China -
Supported by:the National Natural Science Foundation of China, No. 31570978; the National Natural Science Foundation of China for the Youth, No. 51203194
CLC Number:
Cite this article
Zhou Long, He Fan, Wang Lei. Premature senescence of mesenchymal stem cells: causes and preventive measures[J]. Chinese Journal of Tissue Engineering Research, 2018, 22(29): 4721-4728.
share this article
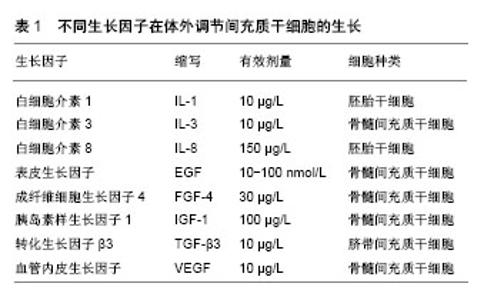
2.1 MSCs早衰的特征 MSCs在经过多次复制传代进入生命晚期后具有一些特征,可与增殖期及静止期细胞区分。这些用以鉴别细胞衰老的特征包括细胞形态变得大而扁平,细胞周期停滞于G1期,β-半乳糖苷酶表达增加[11-12]。无论MSCs来源于何种组织,细胞衰老的特征都是相似的。传代晚期的MSCs的增殖能力下降,细胞周期停滞于G1期,细胞内染色体组正常,无基因突变[13-14]。衰老的MSCs扁平肥大,细胞核和细胞质颗粒固缩,细胞碎片累积。同时由于脂褐质累积,衰老的MSCs肌动蛋白过度表达,细胞黏附性降低,自体荧光水平增加。MSCs衰老时β-半乳糖苷酶染色阳性率接近80%,比正常细胞高出4倍[15]。但值得注意的是,有部分研究显示传代晚期的MSCs的β-半乳糖苷酶染色阳性率不足40%,可能与细胞生长过度抑制有关,这也表明β-半乳糖苷酶染色并非是体外MSCs衰老的有效标记。 衰老的MSCs表达一些与细胞生物学广泛关联的基因,比如与细胞周期、DNA复制、DNA损伤修复、有丝分裂相关基因表达水平的下降[16-17]。另一方面,细胞周期抑制剂(CDKN2b,p16Ink4a)[16]、炎性细胞因子(白细胞介素6)[16]、抗纤维蛋白溶解物(PAI-1)以及促血管新生因子(转化生长因子β1)等基因则过度表达[18],这种改变在一些体细胞中也有类似现象[19]。同时研究发现衰老MSCs内microRNAs表达量也发生改变,microRNAs是一种单股RNA分子,与基因表达的转录修饰密切相关[20]。这些表观遗传变异以往被认为与DNA甲基化过程有关[21],而现在研究表明这种变化导致了细胞衰老[22]。 有研究表明MSCs的成骨分化能力随着细胞寿命的延长逐渐减弱,这与体内年龄相关的骨形成效率降低和骨量丢失的理论一致[23-24]。另一方面,一些研究显示传代后期的MSCs成骨分化潜能有所增加[25]。这种成骨能力的保留与Runx2基因的表达增加有关[26],也有可能与衰老相关的细胞外基质钙沉积有关[25]。无论MSCs对成骨和成脂分化的刺激反应如何,目前的观点倾向于MSCs经过连续传代后成骨和成脂分化能力下降[23,25]。有趣的是,在含有Ⅰ型胶原的环境中培养骨髓MSCs,其成脂分化潜能增加[27]。这种多层分化模式表明基于衰老MSCs的多向潜能变化是一个严格协调的过程,最初是骨-软骨-脂肪三层分化中脂肪分化潜能减弱,而后是骨-软骨两层分化中软骨分化潜能减弱。在这2个体系中,软骨分化潜能逐渐降低,而成骨潜能保持稳定[28]。脂肪分化潜能的中止可能与微囊蛋白1生成增加和过氧化物酶体增殖物激活受体(PPAR?2)信号受累减弱有关[29]。 2.2 MSCs早衰原因 细胞衰老是一个复杂的过程,同时受基因和环境的调控。成纤维细胞传代40-100次后细胞生长停滞,氧化应激诱导DNA链破坏导致端粒缩短,从而细胞出现衰老[30-31]。上皮细胞出现复制停滞的时间更早,复制10-20次后,端粒缩短诱导细胞衰老[32-33]。体外研究表明上皮细胞过早的生长停滞与较差的培养环境有关,培养在最佳的外在环境下,这种现象将会有所改善。在这种最佳的外在环境下,上皮细胞耗尽其寿命后最终以端粒缩短的方式进入衰老[34]。 2.2.1 端粒的作用 端粒是细胞内染色体末端的DNA重复序列,长度相对恒定[35]。传代早期MSCs内端粒长度与供者年龄有关,胚胎来源的MSCs端粒长度为10-11 kb,来源于产后组织的MSCs端粒长度则为7 kb[36]。Baxter等[37]发现来源于产后组织的MSCs端粒长度平均每年以17 bp的速度缩短。然而也有一些研究发现MSCs端粒长度可达11-13 kb,而且长度与供者年龄没有太大关系[38]。 尽管关于MSCs端粒长度没有一致的结论,但MSCs连续多次传代后端粒长度逐渐缩短则是公认的。MSCs每传一次代,端粒将近缩短50-100 bp,与成纤维细胞类似[39]。当MSCs端粒长度缩短至5.8-10.5 kb这个阈值时细胞便开始停止增殖[37],其他体细胞的这个阈值则低于MSCs[40],这表明一些端粒独立事件可能与体外MSCs的衰老有关。 体外细胞衰老时端粒缩短的一个直接证据便是在人端粒酶反转录酶作用下细胞寿命超过其预期的自然极限[41]。端粒酶的主要作用是通过补充染色体末端的重复性序列TTAGGG来弥补端粒在连续复制传代后的缩短[42]。然而大部分体细胞由于端粒酶转录水平的沉默而不具有活性[43]。多数实验认为MSCs的端粒酶活性很低或是无法觉察到其活性[44]。而且,一些研究者发现端粒酶活性具有年龄依赖性[36],另一些学者却认为两者没有关系[38]。MSCs在培养过程中端粒酶的沉默可能与P53的功能和转化生长因子β的连续表达有关[45],它们也是人端粒酶反转录酶的较强抑制剂[43]。 最近几年,大量研究证实MSCs通过基因转染的方式引进人端粒酶反转录酶后复制传代次数倍增(>260代),染色体组型、端粒长度保持正常,衰老表型丧失,但体内外的分化潜能没有减弱[46-48]。胚胎干细胞无限制的自我更新能力也强有力的说明了MSCs的衰老主要与由于端粒酶激活导致的端粒DNA逐渐磨损有关[49]。 2.2.2 氧化应激的作用 氧化应激被认为是DNA损伤和衰老的主要原因[50]。成纤维细胞在生命晚期时,活性氧生成增加,这是由于线粒体新陈代谢紊乱所致,包括细胞色素C氧化酶、烟酰胺腺嘌呤二核苷酸脱氢酶活性增加[51],线粒体膜电位降低,线粒体生物合成增加[52]。氧化应激在细胞衰老中的作用已被研究证实,细胞培养在含氧过高的环境下加快了端粒介导的复制性衰老的进程[53],而经过氧化剂处理的细胞则以非端粒依赖的方式过早衰老[54]。 相对于成纤维细胞和其他体细胞,氧化应激在MSCs生长和衰老中的作用则显得较为复杂。一些间接证据显示活性氧在骨髓和脂肪组织来源MSCs增殖中的作用,它们生长的速率和复制传代的寿命在抗氧化剂(抗坏血酸、白藜芦醇)存在的情况下明显提高[55-56]。其他研究证实MSCs在较少的氧压力环境下寿命得以延长[57],可能与促进MSCs增殖的基因过表达有关,如血管内皮生长因子,肝素结合表皮生长因子等[58]。 Lonergan等[59]研究已发现线粒体在MSCs衰老中扮演重要角色。他们发现在MSCs传代晚期线粒体内三磷酸腺苷增加,耗氧量减少,由此表明细胞的衰老依赖于活性氧的积累。尽管有这些研究发现,氧化应激在MSCs衰老中的作用机制仍有许多未明确,尤其是活性氧积累导致的DNA损伤机制、线粒体功能障碍机制等。 2.3 预防MSCs早衰的措施 2.3.1 生长因子 生长因子可以维持并促进MSCs增殖及分化,但不能减缓端粒缩短的速率,增加端粒酶数量[60-61],见表1。5 μg/L成纤维细胞生长因子2可以在无论有无血清的培养环境下促进MSCs分裂增殖。Mizuta等[62]在兔膝关节损伤模型中发现成纤维细胞生长因子2可以通过促进MSCs增殖提升软骨细胞的修复能力。Ito等[13]也证实成纤维细胞生长因子2通过降低P21、P53、P16基因的表达水平,可以减缓人MSCs长期培养过程中出现的复制性衰老,也有可能是抑制转化生长因子β2 mRNA的表达来减少细胞停滞在G1期的比例。100 μg/L胰岛素生长因子1可通过调节转化生长因子β2促进老化MSCs产生蛋白多糖。"


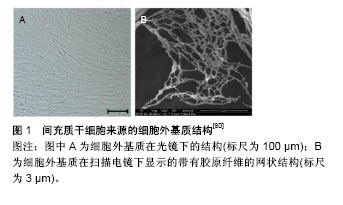
2.3.2 抗氧化剂 N-乙酰半胱氨酸可以保护鼠MSCs免受肿瘤坏死因子诱导的死亡[63]。褪黑素(N-acetyl-5-methoxytryptamine),化学式为N-乙酰基-5-甲氧基色胺,是动物、植物、微生物中的一种天然化合物。在人体中,褪黑素主要由松果体分泌,是一种吲哚胺类激素,具有很强的抗氧化性,可以清除自由基,保护细胞核、线粒体、DNA免受氧化应激损伤[64]。由于褪黑素对细胞有保护作用,因此具备抗衰老的潜能。褪黑素最近被发现在骨髓中也有高水平的分泌[65]。许多研究表明,褪黑素调节各种生理功能,如促进睡眠、调节昼夜节律和神经内分泌活动[66]。褪黑素有众所周知的抗氧化性能,包括清除过多的自由基和增加细胞内抗氧化酶的合成[67]。研究表明,褪黑素通过减少活性氧生成和提高超氧化物歧化酶产生从而保护细胞免受促炎性细胞因子损害[68]。此外,褪黑素已被证明可调控终末分化细胞(关节软骨细胞)的生物功能和MSCs的多向分化[69-70]。然而,褪黑素对早衰MSCs的影响尚未阐明,对SIRT1介导的信号通路争论不一。Yu等[71]发现褪黑素保护心肌缺血再灌注损伤通过以受体依赖的方式激活SIRT1。有令人信服的证据表明褪黑素在MCF-7癌细胞中通过增加P53的乙酰化作用促进细胞周期停滞[72]。因此,褪黑素调控SIRT1及衰老MSCs细胞周期的潜在机制有待进一步研究阐明。褪黑素对MSCs和其他类型细胞遭受氧化应激的保护作用已被广泛研究。Wang等[73]报道褪黑素预处理通过减少胞内活性氧产生及抑制caspase-3活性减弱H2O2诱导的MSCs凋亡。在鼠衰老模型中,褪黑素通过增加BCL-2XL等抗凋亡蛋白的表达改善其寿命[74]。外在的补充褪黑素可以通过减少活性氧的累积抵消促炎性细胞因子致凋亡的影响。目前的结果显示外在补充褪黑素成功保护了MSCs免受H2O2诱导的衰老,并具有浓度依赖性,因为褪黑素减少了β-半乳糖苷酶染色阳性率,增加了细胞增殖能力,下调了P16INK4a的表达水平[75]。一些抗氧化药物也同样具有抗衰老作用,例如内皮细胞外在补充白藜芦醇减弱了氧化应激诱导的衰老,促进细胞增殖[76]。这些研究发现为利用基于MSCs抗氧化应激的细胞理论提供了实验策略。 2.3.3 低氧 21%的氧含量一直被认为是标准的细胞培养环境所需的含氧量,可以最优化的促进成纤维细胞生长,但其他类型的细胞却不适应这种环境。Moussavi-Harami等[77]发现21%的氧环境明显减弱了MSCs的生长速率,这与氧化剂的产生过多有关。MSCs长期培养在低氧环境中,成软骨、成脂分化能力增强,成骨分化能力减弱[78]。研究证实低氧阻止MSCs衰老是通过下调细胞外信号调节激酶表达,上调抑制P16基因表达等实现的[79]。 2.3.4 细胞外基质 干细胞在一个特定的微环境中生长,微环境调控着干细胞如何参与组织再生,维持干细胞自我更新功能的平衡,防止其过度增殖耗尽寿命。细胞外基质作为干细胞微环境的重要组成部分,由各种胶原、蛋白聚糖、纤维蛋白组成,见图1[80]。细胞外基质作为生长因子的储存库,为细胞分化过程的重塑提供了天然信号[81-83]。由于细胞外基质在不同类型细胞中的成分保持相对恒定,来源于不同组织的细胞外基质,如心、肺、脑、肝、脂肪等都可以通过相似的简单生物化学方法获得[84-86]。细胞外基质作为一种细胞支架,支持细胞增殖和分化,Urist[87]研究证实去矿物质的骨基质能够诱导异位骨形成,这归功于骨形态发生蛋白的存在。"
| [1] Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells.Science. 1999;284(5411):143-147. [2] Oreffo RO, Cooper C, Mason C, et al. Mesenchymal stem cells: lineage, plasticity, and skeletal therapeutic potential. Stem Cell Rev. 2005;1(2):169-178. [3] Vidal MA, Walker NJ, Napoli E, et al. Evaluation of senescence in mesenchymal stem cells isolated from equine bone marrow, adipose tissue, and umbilical cord tissue.Stem Cells Dev. 2012;21(2):273-283. [4] Brandl A, Meyer M, Bechmann V, et al. Oxidative stress induces senescence in human mesenchymal stem cells.Exp Cell Res. 2011;317(11):1541-1547. [5] Dumont P, Burton M, Chen QM, et al. Induction of replicative senescence biomarkers by sublethal oxidative stresses in normal human fibroblast.Free Radic Biol Med. 2000;28(3): 361-373. [6] Vacanti V, Kong E, Suzuki G, et al. Phenotypic changes of adult porcine mesenchymal stem cells induced by prolonged passaging in culture.J Cell Physiol. 2005;205(2):194-201. [7] Mezey E.The therapeutic potential of bone marrow-derived stromal cells.J Cell Biochem. 2011;112(10):2683-2687. [8] He H, Liu X, Peng L, et al. Promotion of hepatic differentiation of bone marrow mesenchymal stem cells on decellularized cell-deposited extracellular matrix.Biomed Res Int. 2013; 2013:406871. [9] Kassem M, Marie PJ.Senescence-associated intrinsic mechanisms of osteoblast dysfunctions.Aging Cell. 2011; 10(2):191-197. [10] Li J, Pei M.Cell senescence: a challenge in cartilage engineering and regeneration.Tissue Eng Part B Rev. 2012; 18(4):270-287. [11] Campisi J, d'Adda di Fagagna F.Cellular senescence: when bad things happen to good cells.Nat Rev Mol Cell Biol. 2007; 8(9):729-740. [12] Krtolica A, Campisi J.Cancer and aging: a model for the cancer promoting effects of the aging stroma.Int J Biochem Cell Biol. 2002;34(11):1401-1414. [13] Ito T, Sawada R, Fujiwara Y, et al. FGF-2 suppresses cellular senescence of human mesenchymal stem cells by down-regulation of TGF-beta2.BiochemBiophys Res Commun. 2007;359(1):108-114. [14] Meza-Zepeda LA, Noer A, Dahl JA, et al. High-resolution analysis of genetic stability of human adipose tissue stem cells cultured to senescence.J Cell Mol Med. 2008;12(2):553-563. [15] Wagner W, Horn P, Castoldi M, et al. Replicative senescence of mesenchymal stem cells: a continuous and organized process.PLoS One. 2008;3(5):e2213. [16] Ryu E, Hong S, Kang J, et al. Identification of senescence-associated genes in human bone marrow mesenchymal stem cells.BiochemBiophys Res Commun. 2008;371(3):431-436. [17] Galderisi U, Helmbold H, Squillaro T, et al. In vitro senescence of rat mesenchymal stem cells is accompanied by downregulation of stemness-related and DNA damage repair genes.Stem Cells Dev. 2009;18(7):1033-1042. [18] Sawada R, Ito T, Tsuchiya T.Changes in expression of genes related to cell proliferation in human mesenchymal stem cells during in vitro culture in comparison with cancer cells.J Artif Organs. 2006;9(3):179-184. [19] Shelton DN, Chang E, Whittier PS, et al. Microarray analysis of replicative senescence.Curr Biol. 1999;9(17):939-945. [20] Zamore PD, Haley B.Ribo-gnome: the big world of small RNAs.Science. 2005;309(5740):1519-1524. [21] Fabbri M, Garzon R, Cimmino A, et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B.Proc Natl AcadSci U S A. 2007;104(40):15805-15810. [22] Shibata KR, Aoyama T, Shima Y, et al. Expression of the p16INK4A gene is associated closely with senescence of human mesenchymal stem cells and is potentially silenced by DNA methylation during in vitro expansion.Stem Cells. 2007; 25(9):2371-2382. [23] Banfi A, Muraglia A, Dozin B, et al. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: Implications for their use in cell therapy. ExpHematol. 2000;28(6):707-715. [24] Inoue K, Ohgushi H, Yoshikawa T, et al. The effect of aging on bone formation in porous hydroxyapatite: biochemical and histological analysis.J Bone Miner Res. 1997;12(6):989-994. [25] Digirolamo CM, Stokes D, Colter D, et al. Propagation and senescence of human marrow stromal cells in culture: a simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate.Br J Haematol. 1999;107(2):275-281. [26] Kozhevnikova MN, Mikaelian AS, Paiushina OV, et al. Comparative characterization of mesenchymal bone marrow stromal cells at early and late stages of culturing. IzvAkadNaukSer Biol. 2008;(2):156-162. [27] Mauney JR, Volloch V, Kaplan DL.Matrix-mediated retention of adipogenic differentiation potential by human adult bone marrow-derived mesenchymal stem cells during ex vivo expansion.Biomaterials. 2005;26(31):6167-6175. [28] Muraglia A, Cancedda R, Quarto R.Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model.J Cell Sci. 2000;113 ( Pt 7):1161-1166. [29] Park JS, Kim HY, Kim HW, et al. Increased caveolin-1, a cause for the declined adipogenic potential of senescent human mesenchymal stem cells.Mech Ageing Dev. 2005; 126(5):551-559. [30] Stewart SA, Ben-Porath I, Carey VJ, et al. Erosion of the telomeric single-strand overhang at replicative senescence. Nat Genet. 2003;33(4):492-496. [31] von Zglinicki T.Oxidative stress shortens telomeres.Trends Biochem Sci. 2002;27(7):339-344. [32] Kang MK, Guo W, Park NH.Replicative senescence of normal human oral keratinocytes is associated with the loss of telomerase activity without shortening of telomeres.Cell Growth Differ. 1998;9(1):85-95. [33] Kiyono T, Foster SA, Koop JI, et al. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells.Nature. 1998;396(6706): 84-88. [34] Ramirez RD, Morales CP, Herbert BS, et al. Putative telomere-independent mechanisms of replicative aging reflect inadequate growth conditions.Genes Dev. 2001;15(4): 398-403. [35] Graakjaer J, Christensen R, Kolvraa S, et al. Mesenchymal stem cells with high telomerase expression do not actively restore their chromosome arm specific telomere length pattern after exposure to ionizing radiation.BMC Mol Biol. 2007;8:49. [36] Guillot PV, Gotherstrom C, Chan J, et al. Human first-trimester fetal MSC express pluripotency markers and grow faster and have longer telomeres than adult MSC.Stem Cells. 2007;25(3):646-654. [37] Baxter MA, Wynn RF, Jowitt SN, et al. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion.Stem Cells. 2004;22(5): 675-682. [38] Parsch D, Fellenberg J, Brümmendorf TH, et al. Telomere length and telomerase activity during expansion and differentiation of human mesenchymal stem cells and chondrocytes.J Mol Med (Berl). 2004;82(1):49-55. [39] Bonab MM, Alimoghaddam K, Talebian F, et al. Aging of mesenchymal stem cell in vitro.BMC Cell Biol. 2006;7:14. [40] Allsopp RC, Harley CB.Evidence for a critical telomere length in senescent human fibroblasts.Exp Cell Res. 1995;219(1): 130-136. [41] Bodnar AG, Ouellette M, Frolkis M, et al. Extension of life-span by introduction of telomerase into normal human cells.Science. 1998;279(5349):349-352. [42] Masutomi K, Yu EY, Khurts S, et al. Telomerase maintains telomere structure in normal human cells.Cell. 2003;114(2): 241-253. [43] Horikawa I, Barrett JC.Transcriptional regulation of the telomerase hTERT gene as a target for cellular and viral oncogenic mechanisms.Carcinogenesis. 2003;24(7): 1167-1176. [44] Bernardo ME, Zaffaroni N, Novara F, et al. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms.Cancer Res. 2007;67(19):9142-9149. [45] Serakinci N, Christensen R, Graakjaer J, et al. Ectopically hTERT expressing adult human mesenchymal stem cells are less radiosensitive than their telomerase negative counterpart.Exp Cell Res. 2007;313(5):1056-1067. [46] Kang SK, Putnam L, Dufour J, et al. Expression of telomerase extends the lifespan and enhances osteogenic differentiation of adipose tissue-derived stromal cells.Stem Cells. 2004; 22(7):1356-1372. [47] Shi S, Gronthos S, Chen S, et al. Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression.Nat Biotechnol. 2002;20(6):587-591. [48] Simonsen JL, Rosada C, Serakinci N, et al. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat Biotechnol. 2002;20(6):592-596. [49] Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts.Science. 1998; 282(5391):1145-1147. [50] Passos JF, Von Zglinicki T.Oxygen free radicals in cell senescence: are they signal transducers. Free Radic Res. 2006;40(12):1277-1283. [51] Allen RG, Tresini M, Keogh BP, et al. Differences in electron transport potential, antioxidant defenses, and oxidant generation in young and senescent fetal lung fibroblasts (WI-38).J Cell Physiol. 1999;180(1):114-122. [52] Passos JF, Saretzki G, Ahmed S, et al. Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence.PLoS Biol. 2007;5(5):e110. [53] von Zglinicki T, Saretzki G, Döcke W, et al. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence. Exp Cell Res. 1995;220(1):186-193. [54] Chen QM, Prowse KR, Tu VC, et al. Uncoupling the senescent phenotype from telomere shortening in hydrogen peroxide-treated fibroblasts.Exp Cell Res. 2001;265(2): 294-303. [55] Choi KM, Seo YK, Yoon HH, et al. Effect of ascorbic acid on bone marrow-derived mesenchymal stem cell proliferation and differentiation.J BiosciBioeng. 2008;105(6):586-594. [56] Wang KH, Kao AP, Wangchen H, et al. Optimizing proliferation and characterization of multipotent stem cells from porcine adipose tissue.Biotechnol Appl Biochem. 2008; 51(Pt 4):159-166. [57] 李海生,陈金武,朱玲玲,等.持续低氧增强人骨髓间充质干细胞体外增殖[J].基础医学与临床,2005,25(3):268-271.[58] Ohnishi S, Yasuda T, Kitamura S, et al. Effect of hypoxia on gene expression of bone marrow-derived mesenchymal stem cells and mononuclear cells.Stem Cells. 2007;25(5): 1166-1177. [59] Lonergan T, Brenner C, Bavister B.Differentiation-related changes in mitochondrial properties as indicators of stem cell competence.J Cell Physiol. 2006;208(1):149-153. [60] Barbero A, Grogan S, Schäfer D, et al. Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrogenic capacity.Osteoarthritis Cartilage. 2004;12(6):476-484. [61] Brandl A, Angele P, Roll C, et al. Influence of the growth factors PDGF-BB, TGF-beta1 and bFGF on the replicative aging of human articular chondrocytes during in vitro expansion.J Orthop Res. 2010;28(3):354-360. [62] Mizuta H, Kudo S, Nakamura E, et al. Active proliferation of mesenchymal cells prior to the chondrogenic repair response in rabbit full-thickness defects of articular cartilage.Osteoarthritis Cartilage. 2004;12(7):586-596. [63] Muscari C, Bonafe' F, Farruggia G, et al. Long-term treatment with N-acetylcysteine, but not caloric restriction, protects mesenchymal stem cells of aged rats against tumor necrosis factor-induced death.Exp Gerontol. 2006;41(8): 800-804. [64] Reiter RJ, Acuña-Castroviejo D, Tan DX, et al. Free radical-mediated molecular damage. Mechanisms for the protective actions of melatonin in the central nervous system.Ann N Y Acad Sci. 2001;939:200-215. [65] Tan DX, Manchester LC, Reiter RJ, et al. Identification of highly elevated levels of melatonin in bone marrow: its origin and significance.Biochim Biophys Acta. 1999;1472(1-2): 206-214. [66] Reiter RJ.Pineal melatonin: cell biology of its synthesis and of its physiological interactions.Endocr Rev. 1991;12(2): 151-180. [67] Galano A, Tan DX, Reiter RJ.On the free radical scavenging activities of melatonin's metabolites, AFMK and AMK.J Pineal Res. 2013;54(3):245-257. [68] Liu X, Gong Y, Xiong K, et al. Melatonin mediates protective effects on inflammatory response induced by interleukin-1 beta in human mesenchymal stem cells.J Pineal Res. 2013; 55(1):14-25. [69] Pei M, He F, Wei L, et al. Melatonin enhances cartilage matrix synthesis by porcine articular chondrocytes.J Pineal Res. 2009;46(2):181-187. [70] Hui JH, Li L, Teo YH, et al. Comparative study of the ability of mesenchymal stem cells derived from bone marrow, periosteum, and adipose tissue in treatment of partial growth arrest in rabbit.Tissue Eng. 2005;11(5-6):904-912. [71] Yu L, Sun Y, Cheng L, et al. Melatonin receptor-mediated protection against myocardial ischemia/reperfusion injury: role of SIRT1.J Pineal Res. 2014;57(2):228-238. [72] Proietti S, Cucina A, Dobrowolny G, et al. Melatonin down-regulates MDM2 gene expression and enhances p53 acetylation in MCF-7 cells.J Pineal Res. 2014;57(1):120-129. [73] Wang FW, Wang Z, Zhang YM, et al. Protective effect of melatonin on bone marrow mesenchymal stem cells against hydrogen peroxide-induced apoptosis in vitro.J Cell Biochem. 2013;114(10):2346-2355. [74] Gutierrez-Cuesta J, Tajes M, Jiménez A, et al. Evaluation of potential pro-survival pathways regulated by melatonin in a murine senescence model.J Pineal Res. 2008;45(4):497-505. [75] Zhou L, Chen X, Liu T, et al. Melatonin reverses H2O2 -induced premature senescence in mesenchymal stem cells via the SIRT1-dependent pathway. J Pineal Res. 2015;59(2): 190-205. [76] Kao CL, Chen LK, Chang YL, et al. Resveratrol protects human endothelium from H(2)O(2)-induced oxidative stress and senescence via SirT1 activation.J AtherosclerThromb. 2010;17(9):970-979. [77] Moussavi-Harami F, Duwayri Y, Martin JA, et al. Oxygen effects on senescence in chondrocytes and mesenchymal stem cells: consequences for tissue engineering.Iowa Orthop J. 2004;24:15-20. [78] Jin Y, Kato T, Furu M, et al. Mesenchymal stem cells cultured under hypoxia escape from senescence via down-regulation of p16 and extracellular signal regulated kinase. Biochem Biophys Res Commun. 2010;391(3):1471-1476. [79] Schrobback K, Klein TJ, Crawford R, et al. Effects of oxygen and culture system on in vitro propagation and redifferentiation of osteoarthritic human articular chondrocytes. Cell Tissue Res. 2012;347(3):649-663. [80] Liu X, Zhou L, Chen X, et al. Culturing on decellularized extracellular matrix enhances antioxidant properties of human umbilical cord-derived mesenchymal stem cells.Mater SciEng C Mater Biol Appl. 2016;61:437-448. [81] Badylak SF, Vorp DA, Spievack AR, et al. Esophageal reconstruction with ECM and muscle tissue in a dog model.J Surg Res. 2005;128(1):87-97. [82] Bhrany AD, Beckstead BL, Lang TC, et al. Development of an esophagus acellular matrix tissue scaffold.Tissue Eng. 2006; 12(2):319-330. [83] Brown B, Lindberg K, Reing J, et al. The basement membrane component of biologic scaffolds derived from extracellular matrix.Tissue Eng. 2006;12(3):519-526. [84] Ponce Márquez S, Martínez VS, McIntosh Ambrose W, et al. Decellularization of bovine corneas for tissue engineering applications.Acta Biomater. 2009;5(6):1839-1847. [85] Atala A, Vacanti JP, Peters CA, et al. Formation of urothelial structures in vivo from dissociated cells attached to biodegradable polymer scaffolds in vitro.J Urol. 1992;148(2 Pt 2):658-662. [86] Atala A, Freeman MR, Vacanti JP, et al. Implantation in vivo and retrieval of artificial structures consisting of rabbit and human urothelium and human bladder muscle.J Urol. 1993; 150(2 Pt 2):608-612. [87] Urist MR.The classic: a morphogenetic matrix for differentiation of bone tissue.Clin Orthop Relat Res. 2009; 467(12):3068-3070. [88] Linsley C, Wu B, Tawil B.The effect of fibrinogen, collagen type I, and fibronectin on mesenchymal stem cell growth and differentiation into osteoblasts.Tissue Eng Part A. 2013; 19(11-12):1416-1423. [89] Chen XD.Extracellular matrix provides an optimal niche for the maintenance and propagation of mesenchymal stem cells.Birth Defects Res C Embryo Today. 2010;90(1):45-54. [90] Cukierman E, Pankov R, Stevens DR, et al. Taking cell-matrix adhesions to the third dimension.Science. 2001;294(5547): 1708-1712. [91] Pei M, He F, Kish VL.Expansion on extracellular matrix deposited by human bone marrow stromal cells facilitates stem cell proliferation and tissue-specific lineage potential.Tissue Eng Part A. 2011;17(23-24):3067-3076. [92] Choi HR, Cho KA, Kang HT, et al. Restoration of senescent human diploid fibroblasts by modulation of the extracellular matrix.Aging Cell. 2011;10(1):148-157. [93] He F, Liu X, Xiong K, et al. Extracellular matrix modulates the biological effects of melatonin in mesenchymal stem cells.J Endocrinol. 2014;223(2):167-180. [94] Stein GH, Beeson M, Gordon L.Failure to phosphorylate the retinoblastoma gene product in senescent human fibroblasts. Science. 1990;249(4969):666-669. [95] Wang W, Chen JX, Liao R, et al. Sequential activation of the MEK-extracellular signal-regulated kinase and MKK3/6-p38 mitogen-activated protein kinase pathways mediates oncogenic ras-induced premature senescence.Mol Cell Biol. 2002;22(10):3389-3403. [96] Gray-Schopfer VC, Cheong SC, Chong H, et al. Cellular senescence in naevi and immortalisation in melanoma: a role for p16. Br J Cancer. 2006;95(4):496-505. [97] Beauséjour CM, Krtolica A, Galimi F, et al. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003;22(16):4212-4222. [98] Zhang DY, Wang HJ, Tan YZ.Wnt/β-catenin signaling induces the aging of mesenchymal stem cells through the DNA damage response and the p53/p21 pathway.PLoS One. 2011; 6(6):e21397. [99] Dai SM, Shan ZZ, Nakamura H, et al. Catabolic stress induces features of chondrocyte senescence through overexpression of caveolin 1: possible involvement of caveolin 1-induced down-regulation of articular chondrocytes in the pathogenesis of osteoarthritis.Arthritis Rheum. 2006; 54(3):818-831. [100] Kregel KC, Zhang HJ.An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations.Am J Physiol RegulIntegr Comp Physiol. 2007; 292(1):R18-36. [101] Yao H, Rahman I.Perspectives on translational and therapeutic aspects of SIRT1 in inflammaging and senescence. Biochem Pharmacol. 2012;84(10):1332-1339. [102] Furukawa A, Tada-Oikawa S, Kawanishi S, et al. H2O2 accelerates cellular senescence by accumulation of acetylated p53 via decrease in the function of SIRT1 by NAD+ depletion. Cell Physiol Biochem. 2007;20(1-4):45-54. [103] Tsuji T, Aoshiba K, Nagai A.Cigarette smoke induces senescence in alveolar epithelial cells.Am J Respir Cell Mol Biol. 2004;31(6):643-649. [104] Yao H, Chung S, Hwang JW, et al. SIRT1 protects against emphysema via FOXO3-mediated reduction of premature senescence in mice.J Clin Invest. 2012;122(6):2032-2045. [105] Langley E, Pearson M, Faretta M, et al. Human SIR2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence.EMBO J. 2002;21(10):2383-2396. [106] Chen J, Xavier S, Moskowitz-Kassai E, et al. Cathepsin cleavage of sirtuin 1 in endothelial progenitor cells mediates stress-induced premature senescence.Am J Pathol. 2012; 180(3):973-983. [107] Yuan HF, Zhai C, Yan XL, et al. SIRT1 is required for long-term growth of human mesenchymal stem cells.J Mol Med (Berl). 2012;90(4):389-400. [108] Homma K, Sone M, Taura D, et al. Sirt1 plays an important role in mediating greater functionality of human ES/iPS-derived vascular endothelial cells.Atherosclerosis. 2010;212(1):42-47. [109] Yamashita S, Ogawa K, Ikei T, et al. SIRT1 prevents replicative senescence of normal human umbilical cord fibroblast through potentiating the transcription of human telomerase reverse transcriptase gene.BiochemBiophys Res Commun. 2012;417(1):630-634. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Min Youjiang, Yao Haihua, Sun Jie, Zhou Xuan, Yu Hang, Sun Qianpu, Hong Ensi. Effect of “three-tong acupuncture” on brain function of patients with spinal cord injury based on magnetic resonance technology [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-8. |
| [3] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [4] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [5] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [6] | Shi Yangyang, Qin Yingfei, Wu Fuling, He Xiao, Zhang Xuejing. Pretreatment of placental mesenchymal stem cells to prevent bronchiolitis in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 991-995. |
| [7] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [8] | Fan Quanbao, Luo Huina, Wang Bingyun, Chen Shengfeng, Cui Lianxu, Jiang Wenkang, Zhao Mingming, Wang Jingjing, Luo Dongzhang, Chen Zhisheng, Bai Yinshan, Liu Canying, Zhang Hui. Biological characteristics of canine adipose-derived mesenchymal stem cells cultured in hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1002-1007. |
| [9] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [10] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [11] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| [12] | Duan Liyun, Cao Xiaocang. Human placenta mesenchymal stem cells-derived extracellular vesicles regulate collagen deposition in intestinal mucosa of mice with colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1026-1031. |
| [13] | Pei Lili, Sun Guicai, Wang Di. Salvianolic acid B inhibits oxidative damage of bone marrow mesenchymal stem cells and promotes differentiation into cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1032-1036. |
| [14] | Wang Xianyao, Guan Yalin, Liu Zhongshan. Strategies for improving the therapeutic efficacy of mesenchymal stem cells in the treatment of nonhealing wounds [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1081-1087. |
| [15] | Wang Shiqi, Zhang Jinsheng. Effects of Chinese medicine on proliferation, differentiation and aging of bone marrow mesenchymal stem cells regulating ischemia-hypoxia microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1129-1134. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||