Chinese Journal of Tissue Engineering Research ›› 2018, Vol. 22 ›› Issue (29): 4713-4720.doi: 10.3969/j.issn.2095-4344.0631
Previous Articles Next Articles
CRISPR/Cas9: research advances in hematopoietic stem cell transplantation
Yang Hui, Zhu Jian-wei, Lu Hui-li
- Engineering Research Center of Cell & Therapeutic Antibody, MOE, School of Pharmacy, Shanghai Jiao Tong University, Shanghai 200240, China
-
Revised:2018-05-20Online:2018-10-18Published:2018-10-18 -
Contact:Lu Hui-li, PhD, Associate researcher, Engineering Research Center of Cell & Therapeutic Antibody, MOE, School of Pharmacy, Shanghai Jiao Tong University, Shanghai 200240, China -
About author:Yang Hui, Master candidate, Engineering Research Center of Cell & Therapeutic Antibody, MOE, School of Pharmacy, Shanghai Jiao Tong University, Shanghai 200240, China -
Supported by:the National Natural Science Foundation of China, No. 81273576; Shanghai Scientific and Technological Innovation Project, No. 17431904500
CLC Number:
Cite this article
Yang Hui, Zhu Jian-wei, Lu Hui-li. CRISPR/Cas9: research advances in hematopoietic stem cell transplantation[J]. Chinese Journal of Tissue Engineering Research, 2018, 22(29): 4713-4720.
share this article
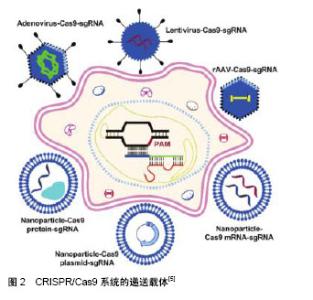
2.1 疾病治疗临床前研究 基于CRISPR/Cas9技术的HSCs基因编辑疗法在血液和免疫系统疾病治疗中具有巨大前景。通过NHEJ和HDR对靶向细胞或者器官进行基因插入和修正、基因敲除、染色体重排甚至是染色体敲除[6],对疾病机制的体内研究和临床试验的发展具有重大意义。这里主要讨论HSCs治疗相关疾病的体外和体内基因编辑。 2.1.1 体外基因编辑 细胞从体内分离并在体外对其进行编辑,之后将编辑后的细胞重新移植回体内,能够消除移植物抗宿主病和移植后免疫抑制的风险。对具有多向分化潜能的HSCs进行编辑,则能够达到长期的药效作用。地中海贫血的病因是α和β珠蛋白链之间比例的失调以及过量的游离α珠蛋白链的作用,使机体产生无效的红细胞并造成溶血。Mettananda等[7]发现当α地中海贫血与β地中海贫血共同遗传时,过量的游离α珠蛋白链显著降低,能够降低病情程度。使用CRISPR/Cas9编辑原代人CD34+细胞模拟MCS-R2(α珠蛋白增强子)缺失的天然突变,能够进一步引起α地中海贫血。在β地中海贫血患者的CD34+细胞中敲除MCS-R2,其分化能力并未受到影响,α珠蛋白表达减少,病理性珠蛋白链失衡也成功校正,脱靶效应也并未检测到[8]。分析发现,经过编辑的CD34+细胞中存在一定比例的长期造血干细胞,说明该方法对治疗地中海贫血症具有较大潜力。 镰状细胞病是由β-珠蛋白基因(b-hemoglobin,HBB)中的单核苷酸多态性(single-nucleotide polymorphism,SNP)引起的隐性遗传疾病。DeWitt等[9]设计包含Cas9蛋白和sgRNA的核糖核蛋白(ribonucleoprotein,RNP)复合物以及单链DNA寡核苷酸供体(single-stranded DNA oligonucleotide donor,ssODN),以有效替代人HSPCs中的镰状细胞病突变。经过编辑的来自镰状细胞病患者的HSPCs产生更少的镰状血红蛋白(hemoglobin,HbA) RNA和蛋白质,并且相应地在分化成有核红细胞时其野生型血红蛋白数目增加。将体外编辑后的人类HSPCs植入免疫缺陷小鼠,在16周内仍然保持稳定。此外,研究表明Cas9 mRNA和Cas9蛋白在进行CRISPR基因组编辑时具有相似的效率[10]。 X连锁慢性肉芽肿病是一种和造血系统相关的单基因疾病,由编码gp91phox的基因CYBB的突变引起,是烟酰胺腺嘌呤二核苷酸磷酸氧化酶2(nicotinamide adenine dinucleotide phosphate oxidase 2,NOX2)的催化中心[11]。De Ravin等[12]使用CRISPR/Cas9修复了患有免疫缺陷病X连锁慢性肉芽肿病患者的HSPCs (CD34+)中的CYBB基因,将其移植至NOD/SCID小鼠中分化成具有成熟的人类髓样和淋巴样细胞长达5个月。全外显子组测序并未检测到脱靶效应,表明这种基因修复策略可能适用于其他慢性肉芽肿病突变和造血系统的单基因疾病。 Gundry等[13]使用CRISPR/Cas9系统直接修饰小鼠和人HSPCs的基因组。使用单独的质粒和非病毒载体将sgRNA单独导入表达Cas9的HSPCs或Cas9-sgRNA的RNP复合物的野生型细胞,结果表明,在小鼠和人类原代HSPCs中分别达到大于60%和75%的基因靶向效率。利用这些技术可以对某些致癌基因进行高效的编辑并评估其功能。 Mandal等[14]在原代人CD4+ T细胞和CD34+ HSPCs中对B2M和CCR5基因进行CRISPR/Cas9靶向,使用单个sgRNA实现了HSPCs中的高效诱变,但在T细胞中突变效率却很低。而双sgRNAs明显改善了2种细胞类型的基因编辑效力。编辑后的HSPCs保留了其多向分化,仅在一个位点观察到低水平的脱靶诱变,这对于以造血干细胞为基础的治疗具有广泛适用性。 Xu等[15]成功地在HSPCs中建立了CRISPR/Cas9基因编辑和非病毒转染系统,实现CCR5敲除并在免疫缺陷小鼠体内产生抗HIV-1能力,具有切割效率高和脱靶效应低的优点,为CCR5基因敲除的HSPCs移植用于HIV治疗提供了依据。经过长期移植的动物模型中,ZFN等方法介导的基因编辑效率通常会降低5-20倍[16],但Xu等[15]发现小鼠体内的CCR5敲除的HSPCs维持了1年以上。 Bohaciakova等[17]利用CRISPR/Cas9获得转基因人类胚胎干细胞。未分化人类胚胎干细胞的简单重复转染导致目的基因p53的表达下调,敲除效率达到82%,并保留了干细胞典型特征,从而显著简化了人类胚胎干细胞中的基因靶向编辑,对基础研究及临床应用前景无限。 2.1.2 体内基因编辑 将基因组编辑组分直接递送至体内以实现对靶向细胞或器官的基因编辑,即体内编辑体系,在多种疾病的治疗中具有独特的效果。Reimer等[18]开发了一种慢病毒CRISPR/Cas9载体,有效诱导人HSPCs在体内转化为MLL(mixed-lineage leukemia)癌症模型,它揭示了人HSPCs中内源性MLL基因重组的致癌效力和局限性,并推动疾病模型向更接近患者的特异性疾病的方向前进。利用CRISPR/Cas9系统实现精确的癌症模型,能够更精准更系统地分析在白血病发生发展过程中的体内平衡机制和关键致癌基因,进行靶向治疗和药物耐药机制的研究。 Suzuki等[19]使用Cas9/sgRNA和同源非依赖性靶向整合(homology-independent targeted integration,HiTi)的方法以靶向小鼠视网膜中的分裂和未分裂细胞,能够实现目的基因的高效整合,若利用这种方法在体内靶向静止的骨髓HSCs进行同源重组同样值得期待。 2.1.3 疾病模型研究 CRISPR/Cas9系统对体内外基因编辑技术的发展有举足轻重的作用,除了应用在疾病治疗上,在与HSCs相关疾病的模型构建等方面也有很多应用[20]。通常血液系统恶性肿瘤是由难以在人体细胞中建模的基因损伤联合驱动的。Tothova等[21]使用CRISPR/Cas9系统编辑成人外周血和婴儿脐血的CD34+ HSPCs,敲除髓系恶性肿瘤相关的一系列基因,移植到免疫缺陷小鼠中建立了克隆造血的癌前病变和模型。这些模型的细胞具有长期、多谱系造血重建及连续移植能力,用于研究人类髓系白血病及癌前病变的遗传复杂性。与之前建立的模型相比[22],该模型能够更准确地预测临床试验中药物的疗效以及对药物干预最敏感的基因型,也证明了通过编辑特定基因或基因簇建立髓系恶性肿瘤模型的可能性。 综上,基于CRISPR/Cas9系统的许多技术在体内外的科学研究甚至在临床中已应用,这些工具显著提高了操纵模式生物基因组的能力,并对HSCs相关的血液及免疫疾病的研究以及个性化医疗提供了新思路。在临床前安全性和致瘤性模型中,HSPCs基因编辑后需要着重研究其造血及脱靶效应,并对其体内外研究模式进行预评估,或者在FDA批准的Ⅰ/Ⅱ期临床试验中对其进行疗效测试。开发基因组编辑体内外的研究计划,为血液相关遗传疾病提供永久治疗,有助于开创干细胞和基因治疗的新领域。 2.2 CRISPR/Cas9的递送方式 CRISPR/Cas9基因编辑组分的递送方式对细胞的编辑效率有很大的影响,因此,提高基因编辑效率一直是研究的主题和方向。CRISPR/Cas9组分能够以DNA、信使RNA(mRNA)和蛋白质的形式[23],通过病毒或者非病毒载体,递送至细胞内特定位置发挥作用,见图2[5]。"
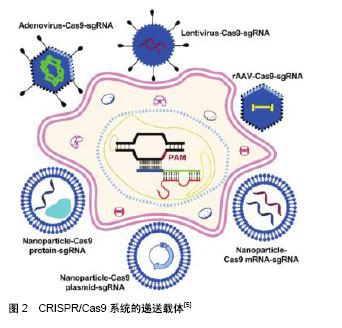
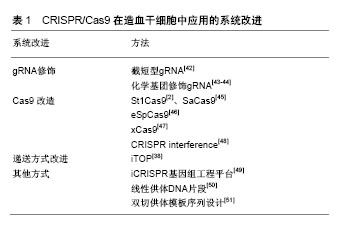
2.2.1 基于病毒载体的递送方式 病毒载体通常用于修饰HSPCs中的基因表达。病毒通过受体介导的内吞作用继而释放病毒基因组影响哺乳动物细胞,体内基因编辑效率高,但由于在宿主基因组中不可预测的整合位点,以及插入突变的可能[24],使其在临床应用中,特别是在干细胞的治疗中受到严格的调控。但相比较于大多数非病毒性载体递送方法而言,病毒载体在基因和细胞治疗中有巨大潜力,以慢病毒、腺病毒、腺相关病毒等为主要代表的病毒载体已被广泛应用于哺乳动物细胞中以进行有针对性的基因组编辑。 慢病毒:慢病毒是一种RNA病毒,它可以整合进入分裂或非分裂期细胞,对难以转化的原代细胞进行有效递送。它装载能力较大,能够达到18 kb,对较大基因组或多基因递送有很大优势[25]。 人类的恶性肿瘤通常由4个或更多基因的突变驱动产生,一般很难用传统方法模拟其遗传复杂性[26]。Heckl等[27]用慢病毒载体将sgRNAs和Cas9结合在一起转染小鼠的HSCs,修改了5个基因,导致了克隆的生长和骨髓恶性肿瘤,因此产生了急性髓系白血病(acute myeloid leukemia,AML)模型。结果表明,慢病毒递送的sgRNA和Cas9的基因组编辑有助于设计一系列更能反映人类疾病复杂性的体内癌症模型。 慢病毒的升级版本,即整合缺陷型慢病毒(integration-deficient lentiviral vectors,IDLVs)[28],对降低脱靶突变率具有很大优势,已成功用于ZFN介导的人造血干细胞和胚胎干细胞的HDR定点整合,借鉴ZFN,将IDLVs应用于CRISPR介导的递送系统中也将会有很大潜力。 腺病毒:腺病毒是一种没有包膜的双链线性DNA病毒,能够感染分裂期细胞和静止细胞。与慢病毒相比,由于腺病毒并未整合进入宿主基因组中,因此脱靶率相对较低[29]。此外,腺病毒包装能力也较大,达到35 kb。然而,腺病毒与慢病毒具有相似的弊端,即可能引发机体的免疫反应、生产成本较高等,这也限制了其在临床上的应用。 Ehrke-Schulz等[30]采用了可临床使用的高容量腺病毒载体(high-capacity adenoviral vectors,HCAdV),去除关键病毒基因从而降低免疫原性,目前尽管还未应用于HSCs,但具有很好的前景。 腺相关病毒:腺相关病毒(adeno-associated virus,AAV)是一种小型单链DNA病毒。在体内表达外源基因稳定,感染分裂和非分裂期细胞,免疫反应和细胞毒性相对较低,促使它在不同的动物模型和人类临床试验中表现出良好的安全性[31]。与慢病毒和腺病毒相比,小于4.7 kb的容量使它的应用受到限制。 针对于镰状细胞性贫血,Dever等[32]使用基于血清型6的重组腺相关病毒载体(recombinant adeno- associated viral vectors of serotype 6,rAAV6)将Cas9 RNP复合物直接递送至HSCs中,结果表明在免疫缺陷小鼠中,其校正突变率和随后移植的整合率极高。在分化成红细胞后,细胞表型和红细胞携氧能力均被激活。 2.2.2 基于非病毒载体的递送方式 在递送CRISPR/Cas9系统中,基因编辑组分通常以mRNA或者Cas9蛋白与sgRNA的复合物的形式递送。由于蛋白质在细胞内能快速降解,RNP能降低与核酸相关的脱靶效应[33]。在这个部分,介绍非病毒载体递送的几类方法,包括电穿孔、脂质体以及其他的一些递送方法[34]。 电穿孔:电穿孔通过提供一个电场使细胞受到高电压脉冲后,在细胞膜表面形成纳米级别的孔径,带负电的DNA和mRNA通过电场相互作用进入细胞从而实现基因编辑组分的递送。与其他非病毒递送方法相比,这种方法不依赖于细胞类型,并且可以高效地将核酸酶递送到难以感染的细胞内,但对细胞能产生较大的杀伤作用[35]。 Wen等[10]在HSPCs中用电转方法递送Cas9 mRNA,培养6 h以充分表达细胞Cas9蛋白,细胞重复进行电穿孔以引入特异性靶向血红蛋白S(hemoglobin-S,HbS)的sgRNA和同源模板;同样利用电转方法递送Cas9蛋白和sgRNA形成的RNP复合物以及同源模板进入HSPCs内,结果表明,Cas9 mRNA和Cas9蛋白进行CRISPR基因组编辑的效率相似。 脂质介导递送:脂质介导的递送方式是利用电荷间的相互作用进行自组装的基因递送方法。脂质体不仅可以用于DNA和RNA的递送,也可以有效递送Cas9蛋白和sgRNA进入细胞达到基因编辑的效果[36]。在CRISPR/Cas9系统中,带正电荷的Cas9蛋白和富负电荷的sgRNA形成富负电荷的RNP复合物。这种复合物可以被阳离子脂质颗粒封装,通过内吞作用和巨胞饮作用递送进入细胞。 Miller等[37]开发了一种利用两性离子氨基酸脂质(zwitterionic amino lipids,ZALs)共递送包括Cas9 mRNA和sgRNAs的长RNA非病毒递送系统,也为HSCs在体内外递送提供了思路。脂质介导递送的效率取决于细胞种类,需要进一步的优化,但无病毒、无质粒的脂质介导的CRISPR/Cas9 RNP递送是CRISPR/Cas9系统应用的理想方法。 2.2.3 其他方式 目前其他领域的纳米粒子、iTOP(induced transduction by osmocytosis and propanebetaine)以及病毒和非病毒方法相结合的方法也在发展之中[38-39],若将它们进一步应用于HSCs领域也是非常值得期待的。 2.3 CRISPR/Cas9在HSCs中应用的系统改进 尽管CRISPR/Cas9系统有很多令人振奋的进展,但其在临床基因治疗中的最终应用仍存在很多挑战,例如免疫原性[40]、脱靶效应等[41]。改进CRISPR/Cas系统中Cas9、gRNA、递送方式、供体同源臂等因素,对增强编辑效率和特异性具有很大帮助,见表1。"
| [1] Wiedenheft B, Sternberg SH, Doudna JA. RNA-guided genetic silencing systems in bacteria and archaea. Nature. 2012;482(7385):331-338.[2] Cong L, Ran FA, Cox D, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121): 819-823.[3] Antoniani C, Romano O, Miccio A. Concise Review: Epigenetic Regulation of Hematopoiesis: Biological Insights and Therapeutic Applications. Stem Cells Transl Med. 2017; 6(12):2106-2114.[4] Dever DP, Porteus MH. The changing landscape of gene editing in hematopoietic stem cells: a step towards Cas9 clinical translation. Curr Opin Hematol. 2017;24(6):481-488.[5] He ZY, Men K, Qin Z, et al. Non-viral and viral delivery systems for CRISPR-Cas9 technology in the biomedical field. Sci China Life Sci. 2017;60(5):458-467.[6] Zuo E, Huo X, Yao X, et al. CRISPR/Cas9-mediated targeted chromosome elimination. Genome Biol. 2017;18(1):224.[7] Mettananda S, Gibbons RJ, Higgs DR. α-Globin as a molecular target in the treatment of β-thalassemia. Blood. 2015;125(24):3694-3701.[8] Mettananda S, Fisher CA, Hay D, et al. Editing an α-globin enhancer in primary human hematopoietic stem cells as a treatment for β-thalassemia. Nat Commun. 2017;8(1):424.[9] DeWitt MA, Magis W, Bray NL, et al. Selection-free genome editing of the sickle mutation in human adult hematopoietic stem/progenitor cells. Sci Transl Med. 2016;8(360): 360ra134.[10] Wen J, Tao W, Hao S, et al. Cellular function reinstitution of offspring red blood cells cloned from the sickle cell disease patient blood post CRISPR genome editing. J Hematol Oncol. 2017;10(1):119.[11] Okura Y, Yamada M, Kuribayashi F, et al. Monocyte/ macrophage-specific NADPH oxidase contributes to antimicrobial host defense in X-CGD. J Clin Immunol. 2015; 35(2):158-167.[12] De Ravin SS, Li L, Wu X, et al. CRISPR-Cas9 gene repair of hematopoietic stem cells from patients with X-linked chronic granulomatous disease. Sci Transl Med. 2017;9(372):eaah 3480.[13] Gundry MC, Brunetti L, Lin A, et al. Highly Efficient Genome Editing of Murine and Human Hematopoietic Progenitor Cells by CRISPR/Cas9. Cell Rep. 2016;17(5):1453-1461.[14] Mandal PK, Ferreira LM, Collins R, et al. Efficient ablation of genes in human hematopoietic stem and effector cells using CRISPR/Cas9. Cell Stem Cell. 2014;15(5):643-652.[15] Xu L, Yang H, Gao Y, et al. CRISPR/Cas9-Mediated CCR5 Ablation in Human Hematopoietic Stem/Progenitor Cells Confers HIV-1 Resistance In Vivo. Mol Ther. 2017;25(8): 1782-1789.[16] Peterson CW, Wang J, Norman KK, et al. Long-term multilineage engraftment of autologous genome-edited hematopoietic stem cells in nonhuman primates. Blood. 2016;127(20):2416-2426.[17] Bohaciakova D, Renzova T, Fedorova V, et al. An Efficient Method for Generation of Knockout Human Embryonic Stem Cells Using CRISPR/Cas9 System. Stem Cells Dev. 2017; 26(21):1521-1527.[18] Reimer J, Knöß S, Labuhn M, et al. CRISPR-Cas9-induced t(11;19)/MLL-ENL translocations initiate leukemia in human hematopoietic progenitor cells in vivo. Haematologica. 2017; 102(9):1558-1566.[19] Suzuki K, Tsunekawa Y, Hernandez-Benitez R, et al. In vivo genome editing via CRISPR/Cas9 mediated homology-I ndependent targeted integration. Nature. 2016;540(7631): 144-149.[20] Cai M, Yang Y. Targeted genome editing tools for disease modeling and gene therapy. Curr Gene Ther. 2014;14(1):2-9.[21] Tothova Z, Krill-Burger JM, Popova KD, et al. Multiplex CRISPR/Cas9-Based Genome Editing in Human Hematopoietic Stem Cells Models Clonal Hematopoiesis and Myeloid Neoplasia. Cell Stem Cell. 2017;21(4):547-555. e8.[22] Frascoli F, Kim PS, Hughes BD, et al. A dynamical model of tumour immunotherapy. Math Biosci. 2014;253:50-62.[23] Glass Z, Lee M, Li Y, et al. Engineering the Delivery System for CRISPR-Based Genome Editing. Trends Biotechnol. 2018; 36(2):173-185.[24] Liu C, Zhang L, Liu H, et al. Delivery strategies of the CRISPR-Cas9 gene-editing system for therapeutic applications. J Control Release. 2017;266:17-26.[25] Rothe M, Modlich U, Schambach A. Biosafety challenges for use of lentiviral vectors in gene therapy. Curr Gene Ther. 2013;13(6):453-468.[26] Cancer Genome Atlas Research Network, Ley TJ, Miller C, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368(22):2059-2074.[27] Heckl D, Kowalczyk MS, Yudovich D, et al. Generation of mouse models of myeloid malignancy with combinatorial genetic lesions using CRISPR-Cas9 genome editing. Nat Biotechnol. 2014;32(9):941-946.[28] Genovese P, Schiroli G, Escobar G, et al. Targeted genome editing in human repopulating haematopoietic stem cells. Nature. 2014;510(7504):235-240.[29] Pjechová M, Hernychová L, Tomašec P, et al. Adenoviral Vectors in Gene Therapy. Klin Onkol. 2015;28 Suppl 2: 2S75-80.[30] Ehrke-Schulz E, Schiwon M, Leitner T, et al. CRISPR/Cas9 delivery with one single adenoviral vector devoid of all viral genes. Sci Rep. 2017;7(1):17113.[31] Petrs-Silva H, Linden R. Advances in recombinant adeno-associated viral vectors for gene delivery. Curr Gene Ther. 2013;13(5):335-345.[32] Dever DP, Bak RO, Reinisch A, et al. CRISPR/Cas9 β-globin gene targeting in human haematopoietic stem cells. Nature. 2016;539(7629):384-389.[33] Song M. The CRISPR/Cas9 system: Their delivery, in vivo and ex vivo applications and clinical development by startups. Biotechnol Prog. 2017;33(4):1035-1045.[34] Sum CH, Wettig S, Slavcev RA .Impact of DNA vector topology on non-viral gene therapeutic safety and efficacy. Curr Gene Ther. 2014;14(4):309-329.[35] He ZY, Men K, Qin Z, et al. Non-viral and viral delivery systems for CRISPR-Cas9 technology in the biomedical field. Sci China Life Sci. 2017;60(5):458-467.[36] Wang M, Zuris JA, Meng F, et al. Efficient delivery of genome-editing proteins using bioreducible lipid nanoparticles. Proc Natl Acad Sci U S A. 2016;113(11):2868-2873.[37] Miller JB, Zhang S, Kos P, et al. Non-Viral CRISPR/Cas Gene Editing In Vitro and In Vivo Enabled by Synthetic Nanoparticle Co-Delivery of Cas9 mRNA and sgRNA. Angew Chem Int Ed Engl. 2017;56(4):1059-1063.[38] D'Astolfo DS, Pagliero RJ, Pras A, et al. Efficient intracellular delivery of native proteins. Cell. 2015;161(3):674-690.[39] Yin H, Song CQ, Dorkin JR, et al. Therapeutic genome editing by combined viral and non-viral delivery of CRISPR system components in vivo. Nat Biotechnol. 2016;34(3):328-333.[40] Dai WJ, Zhu LY, Yan ZY, et al. CRISPR-Cas9 for in vivo Gene Therapy: Promise and Hurdles. Mol Ther Nucleic Acids. 2016; 5:e349.[41] Schaefer KA, Wu WH, Colgan DF, et al. Unexpected mutations after CRISPR-Cas9 editing in vivo. Nat Methods. 2017;14(6):547-548.[42] Fu Y, Sander JD, Reyon D, et al. Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat Biotechnol. 2014;32(3):279-284.[43] Hendel A, Bak RO, Clark JT, et al. Chemically modified guide RNAs enhance CRISPR-Cas genome editing in human primary cells. Nat Biotechnol. 2015;33(9):985-989.[44] Lee K, Mackley VA, Rao A, et al. Synthetically modified guide RNA and donor DNA are a versatile platform for CRISPR-Cas9 engineering. Elife. 2017;6: e25312.[45] Ran FA, Cong L, Yan WX, et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature. 2015;520(7546): 186-191.[46] Slaymaker IM, Gao L, Zetsche B, et al. Rationally engineered Cas9 nucleases with improved specificity. Science. 2016; 351(6268):84-88.[47] Hu JH, Miller SM, Geurts MH, et al. Evolved Cas9 variants with broad PAM compatibility and high DNA specificity. Nature. 2018;556(7699):57-63.[48] Mandegar MA, Huebsch N, Frolov EB, et al. CRISPR Interference Efficiently Induces Specific and Reversible Gene Silencing in Human iPSCs. Cell Stem Cell. 2016;18(4): 541-553.[49] González F, Zhu Z, Shi ZD, et al. An iCRISPR platform for rapid, multiplexable, and inducible genome editing in human pluripotent stem cells. Cell Stem Cell. 2014;15(2):215-226.[50] Paix A, Folkmann A, Goldman DH, et al. Precision genome editing using synthesis-dependent repair of Cas9-induced DNA breaks. Proc Natl Acad Sci U S A. 2017;114(50): E10745-E10754.[51] Zhang JP, Li XL, Li GH, et al. Efficient precise knockin with a double cut HDR donor after CRISPR/Cas9-mediated double-stranded DNA cleavage. Genome Biol. 2017;18(1):35.[52] Zhou H, Zhou M, Li D, et al. Whole genome analysis of CRISPR Cas9 sgRNA off-target homologies via an efficient computational algorithm. BMC Genomics. 2017;18(Suppl 9):826.[53] DiGiusto DL, Cannon PM, Holmes MC, et al. Preclinical development and qualification of ZFN-mediated CCR5 disruption in human hematopoietic stem/progenitor cells. Mol Ther Methods Clin Dev. 2016;3:16067.[54] Tycko J, Myer VE, Hsu PD. Methods for Optimizing CRISPR-Cas9 Genome Editing Specificity. Mol Cell. 2016; 63(3):355-370.[55] Affiliated Hospital to Academy of Military Medical Sciences. Safety of Transplantation of CRISPR CCR5 Modified CD34+ Cells in HIV-infected Subjects With Hematological Malignances.https://clinicaltrials.gov/ct2/show/NCT03164135?term=stem&cond=crispr&rank=1.2017-05-23/2018-03-25.[56] Chinese University of Hong Kong. Identification of Host Factors of Norvovirus Infections in Mini-GutModel. https://clinicaltrials.gov/ct2/show/NCT03342547?term=stem&cond=crispr&draw=2&rank=2.2017-11-17/2018-03-25[57] Sangamo Therapeutics. Product Pipeline. https://www.sangamo.com/product-pipeline/hemoglobi- nopathies. 2018/2018-03-25[58] EDITAS MEDICINE. Diverse Pipeline Across Range of Diseases. http://www.editasmedicine. com/pipeline. 2017-12-09/2018-03-25[59] Intellia THERAPEUTICS. Pipeline. https://www.intelliatx.com/pipeline/. 2018-02-28/2018-03-25.[60] CRISPR THERAPEUTICS. OUR PIPELINE. http://crisprtx.com/our-programs/our-programs.php. 2018/ 2018-03-25.[61] Duardo-Sanchez A. CRISPR-Cas in Medicinal Chemistry: Applications and Regulatory Concerns. Curr Top Med Chem. 2017;17(30):3308-3315. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [3] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [4] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [5] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [6] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [7] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [8] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [9] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [10] | Xing Hao, Zhang Yonghong, Wang Dong. Advantages and disadvantages of repairing large-segment bone defect [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 426-430. |
| [11] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| [12] | Wang Hao, Chen Mingxue, Li Junkang, Luo Xujiang, Peng Liqing, Li Huo, Huang Bo, Tian Guangzhao, Liu Shuyun, Sui Xiang, Huang Jingxiang, Guo Quanyi, Lu Xiaobo. Decellularized porcine skin matrix for tissue-engineered meniscus scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3473-3478. |
| [13] | Mo Jianling, He Shaoru, Feng Bowen, Jian Minqiao, Zhang Xiaohui, Liu Caisheng, Liang Yijing, Liu Yumei, Chen Liang, Zhou Haiyu, Liu Yanhui. Forming prevascularized cell sheets and the expression of angiogenesis-related factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3479-3486. |
| [14] | Liu Chang, Li Datong, Liu Yuan, Kong Lingbo, Guo Rui, Yang Lixue, Hao Dingjun, He Baorong. Poor efficacy after vertebral augmentation surgery of acute symptomatic thoracolumbar osteoporotic compression fracture: relationship with bone cement, bone mineral density, and adjacent fractures [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3510-3516. |
| [15] | Liu Liyong, Zhou Lei. Research and development status and development trend of hydrogel in tissue engineering based on patent information [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3527-3533. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||