Chinese Journal of Tissue Engineering Research ›› 2018, Vol. 22 ›› Issue (16): 2558-2563.doi: 10.3969/j.issn.2095-4344.0829
Previous Articles Next Articles
Morphological observation of broken ends of the mouse tail during wound healing and analysis of cell proliferation active regions
Guo Jing-xu, Wang Hai-tao, Li Shu-wei
- College of Life Sciences of Tarim University, Key Laboratory of Protection & Utilization of Biological Resources in Tarim Basin of Xinjiang Production and Construction Corps, Alar 843300, Xinjiang Uygur Autonomous Region, China
-
Received:2017-12-03Online:2018-06-08Published:2018-06-08 -
Contact:Li Shu-wei, M.D., Professor, College of Life Sciences of Tarim University, Key Laboratory of Protection & Utilization of Biological Resources in Tarim Basin of Xinjiang Production and Construction Corps, Alar 843300, Xinjiang Uygur Autonomous Region, China -
About author:Guo Jing-xu, Master candidate, College of Life Sciences of Tarim University, Key Laboratory of Protection & Utilization of Biological Resources in Tarim Basin of Xinjiang Production and Construction Corps, Alar 843300, Xinjiang Uygur Autonomous Region, China -
Supported by:the National Natural Science Foundation of China, No. 31560685
CLC Number:
Cite this article
Guo Jing-xu, Wang Hai-tao, Li Shu-wei. Morphological observation of broken ends of the mouse tail during wound healing and analysis of cell proliferation active regions[J]. Chinese Journal of Tissue Engineering Research, 2018, 22(16): 2558-2563.
share this article
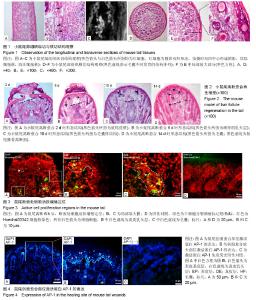
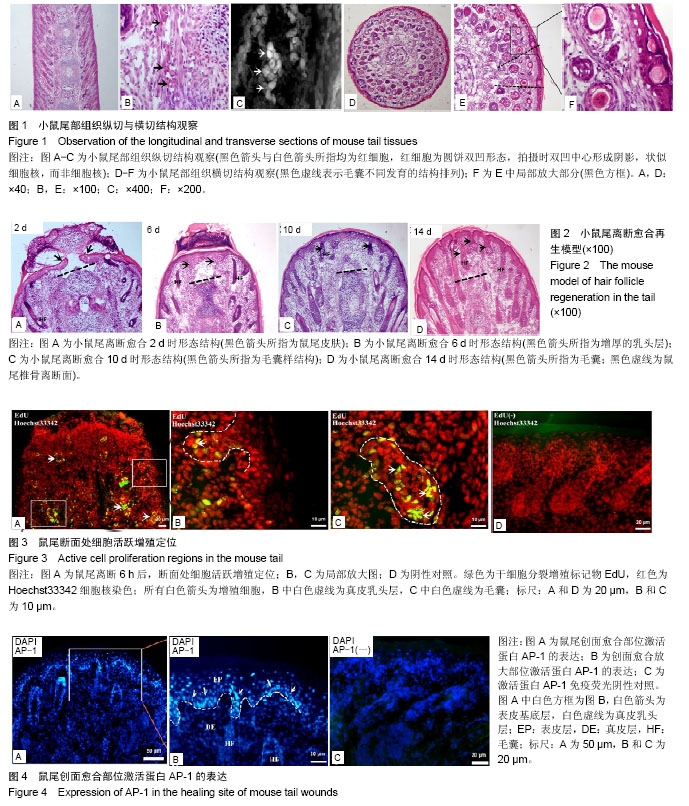
2.1 正常小鼠尾部观察 刚出生的KM幼鼠尾巴柔软光滑红润,随着小鼠生长发育鼠尾外部颜色有粉红变为浅白色,无明显毛发覆盖,大体观察可以明显看出尾部伴行血管。鼠尾离断时,除出血外,还可见拉丝现象,说明鼠尾结构中含有大量胶原蛋白。 2.2 小鼠尾部解剖观察 使用冰冻切片机对所包埋的鼠尾组织进行纵切,所得切片进行苏木精-伊红染色,结果显示(图1A-C):鼠尾部位的毛囊呈现水平45°角对称生长,毛囊与毛囊之间间隙疏密有秩;且纵切图中也可明显分辨出尾椎骨及椎间韧带;经麦克奥迪显微镜放大倍数后,可以明显观察到毛囊与尾椎骨之间的韧带内部含有大量红细胞,说明小鼠尾部组织中血运丰富。使用冰冻切片机对所包埋的鼠尾组织进行横切,所得切片进行苏木精-伊红染色,结果显示(图1D-F):鼠尾部位毛囊的生长分布呈现不是太规则的同心圆状,切片中心为尾椎骨,围绕尾椎骨的外围(前,后,左,右)一共发现4条血管。在横切局部放大图片中,在同一水平切面时,毛囊的发育由外向内依次呈现递减,且外围毛囊有一圈“8”韧带包绕。 2.3 修复过程常规病理染色观察 鼠尾断面修复2 d(图2A)时,鼠尾断面的分泌物及组织液混合形成结痂,覆盖在断面表面,构成一个保护断面的天然屏障,同时鼠尾的表皮往中心聚拢,欲封闭断面,切片染色可以清晰看到尾椎骨的断面(黑色虚线)。鼠尾断面愈合6 d(图2B)时,鼠尾断面的分泌物及组织液混合形成结痂缩水变硬,痂下鼠尾表皮愈合,并发现表皮下的真皮乳头层增厚(黑色箭头),切片染色依旧可以清晰看到尾椎骨的断面(黑色虚线),而且尾椎骨断面与愈合的皮肤之间留有空洞。鼠尾断面愈合 10 d(图2C)时,结痂脱落,尾椎骨断面可以看出,但是空洞缩小,愈合的表皮结构完整,愈合处真皮乳头层厚度及颜色正常,没有残留明显的色素沉着,同时,愈合皮肤处产生新生毛囊样结构(黑色箭头)。鼠尾断面愈合14 d(图2D)时,鼠尾椎骨断面界限不清,创面愈合处皮肤内部被毛囊填充(黑色箭头),毛囊结构完整,维持鼠尾形态与功能。同时也发现鼠尾离断后,断面不仅仅是愈合,也出现了一定皮肤及毛囊再生,愈合部位的鼠尾外部皮肤可以观察到毛发,毛发形态相对正常鼠尾结构部位的毛发无明显差异。 2.4 断面处细胞活跃区定位 鼠尾离断后,立即在鼠尾根部注射2 μL浓度为20 μmol/L EdU进行体内标记鼠尾部位可以进行进行增殖的细胞。6 h后获取鼠尾断端组织,冰冻切片进行细胞增殖荧光染色,结果发现(图3A):鼠尾离断后,鼠尾内部细胞开始进行分裂增殖,放大后,发现表皮的基底层和真皮乳头层也出现了细胞增殖(图3B,白色虚线,白色箭头),细胞的增殖主要出现在毛囊上(图3C,白色虚线,白色箭头),并集中在毛囊球部。PBS代替一抗作为免疫荧光阴性对照(图3D)。 2.5 鼠尾愈合处激活蛋白AP-1表达特点 对鼠尾创面愈合处的皮肤进行激活蛋白AP-1免疫荧光染色鉴定,结果发现:激活蛋白AP-1的表达主要围绕创面愈合的外围(图4A),通过放大后发现,激活蛋白AP-1的表达主要集中在愈合皮肤的表皮基底层(图4B,白色箭头)和真皮乳头层(图4B,白色虚线),真皮乳头层表达激活蛋白AP-1的细胞呈规则线性排列。等量PBS代替一抗进行免疫荧光阴性对照实验(图4C)。"
| [1] Wu Y, Niu Y, Zhong S, et al. A preliminary investigation of the impact of oily skin on quality of life and concordance of self-perceived skin oiliness and skin surface lipids. Int J Cosmet Sci.2013;35(5): 442-447.[2] Iwona Driskell, Feride Oeztuerk-Winder, Peter Humphreys, et al. Genetically Induced Cell Death in Bulge Stem Cells Reveals Their Redundancy for Hair and Epidermal Regeneration. Stem Cells.2015;33(3): 988-998.[3] Marfia G, Navone SE, Di Vito C. Mesenchymal stem cells: potential for therapy and treatment of chronic non-healing skin wounds.Organogenesis.2015;11(4):183-206.[4] Hocking AM. The Role of chemokines in mesenchymal stem cell homing to wounds. Adv Wound Care (NewRochelle).2015;4(11): 623-630.[5] Wang Y, Liu ZY, Zhao Q, et al. Future application of hair follicle stem cells:capable in differentiation into sweat gland cells. Chin Med J (Engl).2013;126 (18): 3545-3552.[6] Purba TS, Haslam IS, Poblet E, et al. Human epithelial hair follicle stem cells and their progeny: current state of knowledge, the widening gap in translational research and future challenges. Bioessays.2014;36 (5): 513-525.[7] Mokos ZB,Mosler EL.Advances in a rapidly emerging field of hair follicle stem cell research. Collegium antropologicum.2014;38 (1): 373-378.[8] Kamachi Y,Kondoh H.Sox proteins: regulators of cell fate specification and differentiation. Development.2013;140(20): 4129-4144.[9] Gao Z, Cox JL, Gilmore JM, et al.Determination of Protein Interactome of Transcription Factor Sox2 in Embryonic Stem Cells Engineered for Inducible Expression of Four Reprogramming Factors.Angie Rizzino J Biol Chem.2012; 287(14):11384-11397.[10] Lien W H,Polak L,Lin M,et al.In vivo transcriptional governance of hair follicle stem cells by canonical Wnt regulators. Nat Cell Biol. 2014;16(2): 179 -190.[11] Rahmani W, Abbasi S, Hagner A, et al. Hair follicle dermal stem cells regenerate the dermal sheath,repopulate the dermal papilla,and modulate hair type. Dev Cell.2014;31(5): 543-558.[12] Rompolas P, Mesa KR, Greco V. Spatial organization within a niche as a determinant of stem-cell fate. Nature.2013;502(7472): 513-518.[13] Leiros GJ, Kusinsky AG, Drago H, et al. Dermal Papilla Cells Improve the Wound Healing Process and Generate Hair Bud-Like Structures in Grafted Skin Substitutes Using Hair Follicle Stem Cells. Stem Cells Transl Med.2014;3(10):1209-1219.[14] Najafzadeh N, Sagha M, Heydari Tajaddod S, et al. In vitro neural differentiation of CD34 (+) stem cell populations in hair follicles by three different neural induction protocols. Cell Dev Biol Anim.2015; 51(2): 192-203.[15] Dunn C,Wiltshire C,MacLaren A,et al.Molecular mechanism and biological functions of c-Jun N-terminal kinase signalling via the c-Jun transcription factor. Cell Signal.2002;14(7): 585-593.[16] Norifumi T, Rajan J, Matthew RL, et al. Hopx expression defines a subset of multipotent hair follicle stem cells and a progenitor population primed to give rise to K6+niche cells. Development. 2013;140(8):1655-1664.[17] Favaro R, Appolloni I, Pellegatta S, et al.Sox2 is required to maintain cancer stem cells in a mouse model of high-grade oligodendro-glioma. Cancer Res.2014;74:1833-1844.[18] Chen PL, Chen WS, Li J, et al.Diagnostic utlity of neural stem and progenitor cell markers nestin and Sox2 in distinguishing nodal melanocytic nevi from metastatic melanomas. Mod Pathol.2013; 26(1):44-53.[19] Jeong-Hyeon Lee, Won-Jae Lee, Ryoung-Hoon Jeon, et al. Development and Gene Expression of Porcine Cloned Embryos Derived from Bone Marrow Stem Cells with Overexpressing Oct4 and Sox2. Cell Reprogram.2014;16(6): 428-438.[20] Hsu YC, Li L, Fuchs E. Emerging interactions between skin stem cells and their niches[J]. Nat Med.2014;20(8):847-856.[21] Lee JH,Koh H,Kim M,et al. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature.2007; 447 (7147):1017-1020.[22] Kuwano M, Horibe Y, Kawashima Y. Effect of collagen cross-linking in collagen corneal shields on ocular drug de-Livery. J Ocul Pharmacol Ther.1997;13(1):31-40.[23] Yamada N, Uchinuma E, Kuroyanagi Y. Clinical evaluation of an allogeneic cultured dermal substitute composed of fibroblasts within a spongy collagen matrix. Scand J Plast Reconstr Surg Hand Surg.1999;33(2):147-154.[24] Das A, Sinha M, Datta S, et al. Monocyte and macrophage plasticity in tissue repair and regeneration. Am J Pathol. 2015; 23(6):235-243.[25] Biswas SK, Chittezhath M, Shalova IN, et al. Macrophage polarization and plasticity in health and disease. Immunol Res. 2012;53(1/2/3):11-24.[26] Lo DD, Zimmermann AS, Nauta A, et al. Scarless fetal skin wound healing update. Birth Defects Res C Embryo Today, 2012, 96(30): 237-247.[27] Akita S, Akino K, Hirano A. Basic fi broblast growth factor in scarless wound healing. Adv Wound Care (New Rochelle). 2013; 2(2):44-49.[28] Green SA, Simoes CM, Bronner ME. Evolution of vertebrates as viewed from the crest. Nature. 2015;520(7548): 474-482.[29] Mozafari S, Laterza C, Roussel D, et al. Skin- derived neural precursors competitively generate functional myelin in adult demyelinated mice. J Clin Invest.2015;125(9): 3642-3656.[30] Sato H, Ebisawa K, Takanari K, et al. Skin-derived precursor cells promote wound healing in diabetic mice.Ann Plast Surg.2015; 74(1):114-120.[31] Ochalek M, Hleiss RS, Wohl AB J, et al. Characterization of lipid model membranes designed for studying impact of ceramide species on drug diffusion and penetration.Eur J Pharm Biopharm. 2012;81(1):113-120.[32] Rahmani W, Abbasi S, Hagner A, et al. Hair follicle dermal stem cells regenerate the dermal sheath, repopulate the dermal papilla,and modulate hair type. Dev Cell.2014;31(5): 543-558.[33] Shu B, Xie JL, Xu YB, et al. Directed differentiation of skin-derived precursors into fibroblast-like cells. Int J Clin Exp Pathol.2014; 7(4):1478-1486.[34] Mehrabi M,Mansouri K,Hosseinkhani S,et al.Differentiation of human skin-derived precursor cells into functional islet-like insulin-producing cell clusters. In Vitro Cell Dev Biol Anim.2015;51 (6):595-603.[35] Thangapazham RL,Darling TN,Meyerle J.Alteration of skin properties with autologous dermal fibroblasts.Int J Molecul Sci. 2014;15(5):8407-8427.[36] Huang Z, Zhen Y, Yin W, Ma Z, Zhang L. Shh promotes sweat gland cell maturation in three-dimensional culture. Cell Tissue Banking.2016;17(2):317-325.[37] Boekema B,Boekestijn B,Breederveld RS.Evaluation of saline,RPMI and DMEM/F12 for storage of split-thickness skin grafts.Burns.2015;41(4):848-852.[38] Zhang C, Chen Y, Fu X. Sweat gland regeneration after burn injury: Is stem cell therapy a new hope. Cytotherapy.2015; 17(5):526-535.[39] Zhao Z, Xu M, Wu M, et al. Direct reprogramming of human fibroblasts into sweat gland-like cells. Cell Cycle.2015;14(21): 3498-3505.[40] Joannides A, Gaughwin P, Schwiening C, et al. Efficient generation of neural precursors from adult human skin:astrocytes promote neurogenesis from skin-derived stem cells. Lancet. 2004; 364(9429):172-178.[41] Ikeda R, Ling J, Cha M, et al. In situ patch-clamp recordings from Merkel cells in rat whisker hair follicles, an experimental protocol for studying tactile transduction in tactile-end organs. Mol Pain. 2015;11(23):15-22.[42] Bruns I,Lucas D,Pinho S,et al.Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion . Nature Med.2014;20(11):1315-1320.[43] Andersen DC,Skovrind I,Christensen ML, et al. Stem cell survival is severely compromised by the thymidine analog EdU, an alternative to BrdU for proliferation assays and stem cell tracing. Anal Bioanal Chem.2013;405(29):9585-9591. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Li Jing, Xie Jianshan, Cui Huilin, Cao Ximei, Yang Yanping, Li Hairong. Expression and localization of diacylglycerol kinase zeta and protein kinase C beta II in mouse back skin with different coat colors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1196-1200. |
| [3] | Li Cai, Zhao Ting, Tan Ge, Zheng Yulin, Zhang Ruonan, Wu Yan, Tang Junming. Platelet-derived growth factor-BB promotes proliferation, differentiation and migration of skeletal muscle myoblast [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1050-1055. |
| [4] | Liu Cong, Liu Su. Molecular mechanism of miR-17-5p regulation of hypoxia inducible factor-1α mediated adipocyte differentiation and angiogenesis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1069-1074. |
| [5] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [6] | Ma Zetao, Zeng Hui, Wang Deli, Weng Jian, Feng Song. MicroRNA-138-5p regulates chondrocyte proliferation and autophagy [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 674-678. |
| [7] | Ma Binxiang, He Wanqing, Zhou Guangchao, Guan Yonglin. Triptolide improves motor dysfunction in rats following spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 701-706. |
| [8] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [9] | Wang Yujiao, Liu Dan, Sun Song, Sun Yong. Biphasic calcium phosphate loaded with advanced platelet rich fibrin can promote the activity of rabbit bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 504-509. |
| [10] | Zhou Jihui, Yao Meng, Wang Yansong, Li Xinzhi, Zhou You, Huang Wei, Chen Wenyao. Influence of novel nanoscaffolds on biological behaviors of neural stem cells and the related gene expression [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 532-536. |
| [11] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [12] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [13] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [14] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [15] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||