Chinese Journal of Tissue Engineering Research ›› 2018, Vol. 22 ›› Issue (16): 2549-2557.doi: 10.3969/j.issn.2095-4344.0237
Previous Articles Next Articles
Variations of sodium chloride concentration affect conformational dynamics of human neuronal calcium sensor-1 protein: a molecular dynamics simulation study
Zhu Yu-zhen1, Li Yun-xiang1, Cai Hao1, Zhang Qing-wen2
- 1Physical Education College, Shanghai Normal University, Shanghai 200234, China; 2Shanghai University of Sport, Shanghai 200438, China
-
Received:2018-03-12Online:2018-06-08Published:2018-06-08 -
Contact:Cai Hao, Professor, Physical Education College, Shanghai Normal University, Shanghai 200234, China -
About author:Zhu Yu-zhen, Ph.D., Physical Education College, Shanghai Normal University, Shanghai 200234, China -
Supported by:the Young Teacher Training Project of Shanghai Universities, No. A-9103-17-041331; the Postgraduate Education Innovative Research Project of Shanghai University of Sport, No. yjscx2015003
CLC Number:
Cite this article
Zhu Yu-zhen1, Li Yun-xiang1, Cai Hao1, Zhang Qing-wen2. Variations of sodium chloride concentration affect conformational dynamics of human neuronal calcium sensor-1 protein: a molecular dynamics simulation study[J]. Chinese Journal of Tissue Engineering Research, 2018, 22(16): 2549-2557.
share this article

2.1 在不同浓度NaCl溶液中,人类神经元钙传感蛋白整体结构基本保持完整,但随着NaCl浓度的升高,局部结构L2(氨基酸G133-P145)的柔性提高了 为了解人类神经元钙传感蛋白在不同浓度NaCl水溶液中的整体结构特征,以能量最小化结构(iWT-1和iWT-2)为参考结构,计算蛋白核心结构(氨基酸E11-K174)、N段(氨基酸E11-S93)、C段(氨基酸D98-K174)在3种不同浓度NaCl水溶液中的均方根偏差(RMSD)值,见图2。均方根偏差用来比较模拟过程中两个结构(参考结构与被计算结构)随时间变化的差异。通常情况下,均方根偏差值越小,表示两个结构间的差别越小,表明该结构比较稳定。图2(a,d)中黑色曲线是人类神经元钙传感蛋白核心结构在0.1 mol/L NaCl浓度水溶液中的均方根偏差随模拟时间的变化曲线。结果显示,核心结构的均方根偏差在前50 ns快速增加,RMSD值经轻微波动,在100 ns之后两个结构分别逐渐保持在0.33 nm(a)和 0.42 nm(d),表明人类神经元钙传感蛋白核心结构在 0.1 mol/L NaCl浓度的水溶液中,在最后400 ns模拟过程中保持了其基本结构;N段的均方根偏差值(b,e的黑色曲线)和WT1的C段(c中的黑色曲线)在0.1 mol/L NaCl浓度的水溶液中的变化曲线与核心结构的均方根偏差有类似的变化趋势,在500 ns模拟过程中,分别逐渐稳定在0.29 (b), 0.40 (e) and 0.31 nm (c),而WT2体系C段的均方根偏差(f中的黑色曲线)在0-320 ns期间有较大的波动,最终逐渐稳定在0.35 nm。 在200-500 ns模拟期间,神经元钙传感蛋白在0.3 mol/L NaCl浓度中核心结构和N段的均方根偏差值(图2 a,b,d,e中的红色曲线)比其在0.1 mol/L NaCl浓度中的RMSD值小,最终逐渐分别稳定在大约0.28(a),0.20(b),0.35(d), 0.27 nm(e);0.3 mol/L NaCl浓度下,第一个结构(WT1)C段均方根偏差曲线与其在0.1 mol/L NaCl浓度中的曲线基本重合,最终稳定在0.3 nm(c),而第二个结构(WT2)在模拟过程中,C段的均方根偏差曲线波动较大,比其在0.1 mol/L NaCl浓度中的均方根偏差值大,最终稳定在大约0.37 nm(f)。 神经元钙传感蛋白WT1在1 mol/L NaCl浓度(蓝色曲线)中核心结构、N段和C段的均方根偏差曲线与其在0.1 mol/L NaCl浓度中的曲线基本重合,相对于在0.3 mol/L NaCl浓度中,WT2在1 mol/LNaCl浓度中的均方根偏差曲线与其在0.1 mol/L NaCl浓度中的曲线更接近。 为探索在500 ns计算机动力学模拟中,神经元钙传感蛋白C段结构变化的详细过程,又计算了蛋白随时间变化的二级结构,见图3。二级结构是以蛋白结构主链中氨基之间的氢键来定义,既能定性观察二级结构随时间的变化情况,也能做定量分析。二级结构中各组成部分的含量不同,蛋白结构构型、稳定性等均不同。 图3显示,在人类神经元钙传感蛋白二级结构中,出现较多、也是构成蛋白结构的重要成分包括:α螺旋(蓝色)、弯曲结构(绿色)、转角结构(黄色)、β折叠(红色)。其中,α螺旋是维持蛋白结构稳定的最重要因素,螺旋百分比越高,结构相对越稳定;反之,结构相对不稳定。在人类神经元钙传感蛋白结构中,螺旋部分折叠-解折叠过程主要发生在环L2(氨基酸G133-P145)和L3(氨基酸D176-V190),而图(d)和(f)中折叠-解折叠的位置主要发生在蛋白C端尾部L3。图(e)中,环L2和L3上形成了新的α螺旋,此螺旋分别在190-500和0-500 ns模拟过程中处于解折叠-折叠状态,而环L2上的α螺旋在0.1和1 mol/L NaCl浓度中消失了,图(f)中,环L3上的螺旋只在220-500 ns模拟中出现,但人类神经元钙传感蛋白在不同浓度NaCl水溶液中都很大程度地保持了其二级结构,表明图2(f)中C段较大的均方根偏差值(> 0.5 nm)可能是因为蛋白的三级结构进行了一定程度的重组。 为继续找寻引起蛋白结构变化的因素,计算了反映蛋白结构稳定的氢键数目(Number of H-bond)和螺旋百分比(Percentage of helix),见图4。在模拟过程中,蛋白结构中的氢键数目和螺旋百分比都是用来描述蛋白结构稳定与否的指标,通常氢键数目越多,螺旋百分比越高,结构相对越稳定。图4显示,在1 mol/L NaCl溶液中(蓝色曲线),人类神经元钙传感蛋白结构中氢键数目(a,b)的平均值在3种不同浓度中最低,分别是73和77;而在0.3 mol/L NaCl溶液中(红色曲线)氢键的平均值最大,分别大约为83和85;在0.1 mol/L NaCl溶液中(黑色曲线)氢键的平均值分别为78和81。 人类神经元钙传感蛋白结构中螺旋百分比(c,d)也表现出类似的曲线。在1 mol/L NaCl溶液中,WT1结构中螺旋百分比的平均数目为0.44和0.47,WT2结构中大约为0.53和0.56,比蛋白在0.1 mol/L和0.3 mol/L NaCl溶液中的螺旋百分比低。WT1在0.1 mol/L和0.3 mol/L NaCl溶液中的螺旋百分比曲线重合,平均数量大约为0.53和0.56;WT2在0.1 mol/L NaCl溶液中的螺旋百分比平均数量分别为0.53和0.56,在0.3 mol/L NaCl溶液中大约为0.57。 氢键数目的增减变化代表氢键的形成和破坏,是螺旋形成折叠和解折叠的前提条件。二级结构图3(c)显示,WT1结构在模拟过程中,螺旋H2(氨基酸E24-F34)、H4(氨基酸T62-F72)、H5(氨基酸F82-S93)和H8(氨基酸E146-M155)出现折叠和解折叠现象。如在20-500 ns期间,螺旋H4受到破坏,大部分解折叠,形成3-螺旋(灰色)、转角结构(黄色)或者是弯曲结构(绿色);在0-400 ns期间,螺旋H2受到"


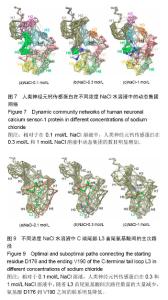
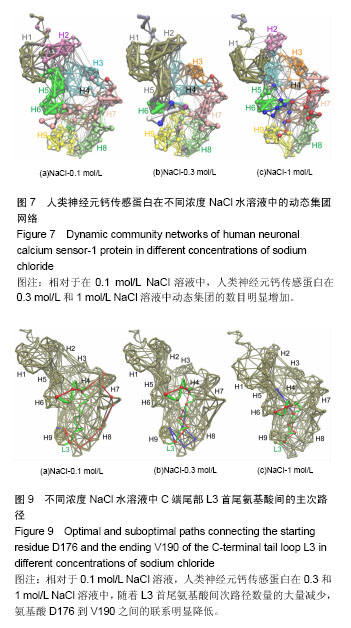
破坏,部分解折叠,形成转角结构(黄色),而在400 ns之后,又重新折叠成螺旋。二级结构图3(f)显示,螺旋部分解折叠和形成折叠的动态过程主要在螺旋H8(氨基酸E146-M155)和环L3(氨基酸D176-V190)上,在0-385 ns期间,螺旋H8受到破坏,部分解折叠,形成转角结构(黄色)或者是弯曲结构(绿色),而在t=385 ns之后又重新折叠成螺旋。C端尾部L3上新形成的螺旋在1 mol/L NaCl溶液中折叠和解折叠过程与其在0.1和0.3 mol/L NaCl溶液中不同,并非从模拟开始就形成螺旋,而是在t=220 ns之后才形成螺旋。这些数据表明,人类神经元钙传感蛋白在1 mol/L NaCl溶液中比其在0.1和0.3 mol/L NaCl溶液中更不稳定,但二级结构还能基本保持完整。 掌握了人类神经元钙传感蛋白在不同NaCl浓度溶液中的整体结构特征后,为进一步了解蛋白在3种不同浓度NaCl溶液中局部结构的动力学特征,以模拟轨迹中200-500 ns的平均结构为参照,计算了核心结构(氨基酸 E11-K174)中每个氨基酸的柔性(Root mean square fluctuation,RMSF),见图5。蛋白柔性曲线既可以从微观视角观察蛋白各原子位置的涨落;也能够宏观把握蛋白平均结构的涨落。 图5显示,除个别位置外,人类神经元钙传感蛋白在0.3和1 mol/L NaCl溶液中(红色和蓝色曲线)的柔性不同程度增加,均大于其在0.1 mol/L NaCl溶液(黑色曲线)中的柔性,尤其是环L2(氨基酸G133-P145)位置明显比其他部分的柔性大,表明在人类神经元钙传感蛋白结构中,环L2局部位置上具有最大的柔性,这一结果与在R102Q突变体及截去L3结构中观察的结果一致[17,30];而且,环L2在0.3 mol/L和1 mol/L NaCl溶液中柔性增加明显,表明在人类神经元钙传感蛋白结构中,环L2的柔性不仅对自身结构的微小变化敏感,而且周围环境NaCl浓度的变化也容易引起其柔性的改变。 2.2 溶液中NaCl浓度升高使蛋白整体结构和局部的疏水口袋体积增大 神经元钙传感蛋白通过疏水口袋中的结合位点与配体结合,发挥其功能。因此,疏水口袋及其内部结合位点的暴露程度,能够影响蛋白功能的发挥。为了解蛋白整体结构体积和局部疏水口袋的大小,分别计算了两个体系的回旋半径(Radius of gyration,Rg)和溶剂可及表面积(Solvent accessible surface area,SASA),以进行定量分析。回旋半径是用于度量蛋白结构的紧凑度,反映蛋白的体积和形状,同一体系的回旋半径越大,说明结构发生了膨胀,体积增大;溶剂可及表面积的大小代表蛋白疏水性的强弱。图6显示了人类神经元钙传感蛋白在不同浓度NaCl溶液中的回旋半径和疏水口袋溶剂可及表面积的概率分布函数(Probability distribution function,PDF)。蛋白两个结构WT1(a)和WT2(b)在3种不同浓度NaCl溶液中的回旋半径平均值分别为1.72和1.74 nm(黑色曲线),1.81和1.78 nm(红色曲线),1.73和1.76 nm(蓝色曲线)。从图中可以看出,蛋白在0.3 mol/L和1 mol/L NaCl溶液中的回旋半径比其在0.1 mol/L NaCl溶液中的大,表明NaCl浓度升高,蛋白整体结构膨胀,体积增大,而且在0.3 mol/L NaCl溶液中增幅最大。 蛋白疏水口袋内部溶剂可及表面积的曲线分布也有类似的趋势,在3种NaCl浓度中的曲线明显不同。在0.1 mol/L NaCl溶液中,蛋白两个结构WT1(c)和WT2(d)溶剂可及表面积的峰值分别为15.4和15.5 nm2,而在0.3 mol/L和 1 mol/L NaCl溶液中曲线右移,分别为17.0和16.1 nm2(红色曲线)和15.8和15.9 nm2(蓝色曲线),大于其在0.1 mol/L NaCl溶液中的溶剂可及表面积,这表明随着蛋白整体结构的膨胀,局部疏水口袋的体积也同时增大,而且在0.3 mol/L NaCl溶液中增加幅度最大。这样可能暴露出疏水口袋中更多的结合位点,更利于配体结合,发挥功能。 2.3 溶液中NaCl浓度升高,蛋白N段和C段内部氨基酸的联系加强,两段之间氨基酸的关联减弱 分子动力学模拟轨迹中各个氨基酸之间的关联可以用动态集团网络显 示[29,31],从动态网络图中能直观地看到受影响的氨基酸,也能分辨各个氨基酸所从属的集团,以及氨基酸相互之间联系的强弱。为进一步把握计算机模拟过程中人类神经元钙传感蛋白的整体和局部结构特性,计算了蛋白在3种不同浓度NaCl溶液中结构特性的动态集团网络图。 图7中的球体是每个氨基酸以Cα原子为中心形成的点,点与点之间的连线是在整个模拟过程中,一对氨基酸之间的距离≤0.45 nm,至少有75%以上相互作用的两点形成。连线的粗细对应相应的权重,连线越粗,权重越大,表明两点之间的联系越密切,相互作用越强。在蛋白动态网络中,用两点间平均关联时间的长短定义氨基酸之间相互作用的大小及联系的强弱,称为集团(community)[32],图7中同一颜色的球体表示在同一个集团中,具有较强较多的联系;不同集团的点之间的相互作用较小,联系较弱。图7显示,人类神经元钙传感蛋白在0.1,0.3,1 mol/L浓度NaCl溶液中动态集团的数目明显不同,分别包括9个(a)、13个(b)和12个(c)集团,表明NaCl浓度改变了氨基酸之间的联系和相互作用。另外蛋白N段和C段内部及两段间的联系也明显不同。在0.1 mol/L浓度NaCl溶液中,两段之间的联系紧密,尤其螺旋H5(属于N段)和H6(属于C段)属于同一集团(绿色),表明N段和C段之间的跨段联系较多较强;而随着NaCl浓度的升高,蛋白N段和C段内部的相互作用增强,蛋白某些集团内氨基酸的联系加强,如0.3 mol/L中螺旋H1、H2和H5,1 mol/L中螺旋H1和H2联系较多较强;同时两段间的联系减弱,表明NaCl浓度能够改变蛋白氨基酸之间的相互作用和联系。结果显示,虽然蛋白在0.3 mol/L和1 mol/L浓度NaCl溶液中的二级结构很大程度地保持完整,但氨基酸之间的联系及动态网络均发生改变,这可能是人类神经元钙传感蛋白在NaCl浓度微小变化时构象动力学变化的原因。这些结果与作者前期对溶液温度升高蛋白结构变化和截去C端尾部L3的研究结果一致[13,30],表明N段和C段之间联系的增强可能对蛋白保持整体结构非常重要。 需要注意的是C端尾部L3上氨基酸动态集团的改变。在0.1 mol/L浓度NaCl溶液中,环L3和螺旋H7部分氨基酸、环L 2之间的联系较多,相互作用较为集中;而在0.3 mol/L和1 mol/L NaCl溶液中,L3上的氨基酸分别与属于5个集团和4个集团的其他氨基酸相联系,相互作用相对分散,表明蛋白周围溶液中NaCl浓度不同明显改变了环L3上氨基酸间的相互作用。 2.4 溶液中NaCl浓度升高,环L3首尾氨基酸间的距离减小,更倾向于蜷缩在疏水口袋中 蛋白C端尾部L3与疏水口袋的位置关系直接关系到蛋白功能的发挥。图3二级结构(d,e,f)显示,在500 ns模拟过程中,WT2结构中C端尾部L3上出现一个a螺旋结构,在不同NaCl浓度溶液中均出现解折叠和折叠过程。为定量描述L3在疏水口袋中的位置,计算了200-500 ns模拟时间段内L3首尾氨基酸D176和V190之间的Ca-Ca距离,见图8。 在0.1 mol/L NaCl溶液中,氨基酸D176和V190之间距离的峰值分别为2.3和1.8 nm(黑色曲线);在0.3 mol/L和 1 mol/L NaCl溶液中,氨基酸D176和V190之间距离减小,曲线左移,峰值分别位于1.1和1.0 nm(红色曲线)、1.4和 1.3 nm(蓝色曲线)。这表明环L3在0.1 mol/L NaCl溶液中可能主要处于伸展状态,占据疏水口袋内部更多的位置;NaCl浓度升高,L3更倾向于蜷缩在疏水口袋内,占据部分疏水口袋,暴露出更多的结合位点,这一结果与图3显示的结果一致。神经元钙传感蛋白主要通过疏水口袋内部的结合位点结合配体,发挥多种生理功能[33-35],扩张的疏水口袋(图4)和L3在疏水口袋位置的改变揭露了疏水口袋的暴露程度不同。溶液中NaCl浓度增加导致疏水口袋扩张(图4)、L3蜷缩在疏水口袋内,可能暴露出其内部更多的结合位点,有利于配体结合,发挥生理功能,尤其在0.3 mol/L NaCl溶液中更为明显。 2.5 溶液中NaCl浓度升高,L3首尾氨基酸D176和V190之间的联系明显减弱 蛋白中两个氨基酸之间联系的强弱可以用两者之间相互联系的主路径和次路径进行评价[31]。主路径是两氨基酸之间的最短路径;比主路径稍长、可供选择的多条线称为次路径[29]。为直观地显示C端尾部L3首尾氨基酸之间的联系,计算了不同浓度下L3首尾氨基酸(D176和V190)间的主次路径,见图9。其中C端尾部L3用绿色表示,主路径用红色表示,为显示清晰,图9中只显示一条次路径,用蓝色表示。 在0.1 mol/L NaCl溶液中,L3首尾氨基酸D176到V190之间有6 081条次路径,而在0.3 mol/L和1 mol/L NaCl溶液中,分别只有2条和20条次路径,表明在0.1 mol/L NaCl溶液中氨基酸D176到V190之间有很强的联系,但溶液中NaCl浓度升高,两个氨基酸之间的次路径削减异常严重,联系明显减弱,尤其在0.3 mol/L NaCl溶液中。图9显示,在0.1 mol/L NaCl溶液中,主路径和次路径上的氨基酸主要包括螺旋H9、H8和环L2、L3;但NaCl浓度升高,主次路径上的氨基酸主要在C端尾巴L3上,表明随着次路径数量的大量减少,氨基酸D176到V190之间联系的强度明显降低,可能改变了环L3在疏水口袋内的状态。 2.6 溶液中NaCl浓度升高,盐桥的数量和百分比明显减少,尤其是C段 盐桥是由蛋白结构中正负电荷侧链质心之间的距离≤0.4 nm形成[32],盐桥无论埋藏还是暴露在水溶液中[33-38],对蛋白结构的稳定都起到至关重要的作 用[39-40]。蛋白中盐桥数目越多,结构相对越稳定;盐桥形成的比例越大,这对盐桥在维持结构稳定中起到的作用越大。人类神经元钙传感蛋白是高电荷蛋白,结构中190个氨基酸中包括24个正电荷和33个负电荷,为探讨不同NaCl浓度下的盐桥形成是否受到影响,计算了0-500 ns模拟中正负电荷形成盐桥的概率,图10显示了蛋白结构中最受影响的盐桥。 图10显示,在3种浓度NaCl水溶液中,人类神经元钙传感蛋白盐桥形成的百分比和数目均明显不同。在 0.1,0.3和1 mol/L NaCl溶液中,形成盐桥的最高百分比逐渐降低,分别为80%,60%,50%,形成盐桥的数目也明显减少,尤其在0.3 mol/L中减少更明显,表明NaCl浓度的微小变化降低了盐桥形成的概率和数量,改变了的盐桥网络可能会影响蛋白结构的稳定性,可能是蛋白构象变化的关键因素。 其中,最受影响的盐桥既包括N段和C段内部的盐桥,N段最受影响的段内盐桥包括K9-E11、E15-R18、K36-D37、D44-R79等,C段最受影响的段内盐桥包括E99-R102、E140-R151、D161-K163和E171-K174等;也包括N段和C段之间的盐桥,如K63-D123、K63-D126、E74-K106等。现有研究结果与作者之前对溶液温度升高、突变体R102Q、截去C端尾部等结果比较发现,盐桥是保持蛋白整体构象特征的重要因素[13,17,30],尤其是盐桥E15-R18、E15-K19、D44-R79、E26-R94、E99-R102、R118-D150、E142-R151和K63-D123在一项计算机模拟研究和前面的研究中也进行过报道,表明上述盐桥在保持人类神经元钙传感蛋白整体结构中可能起到重要作用[41]。 有趣的是溶液中NaCl浓度从0.1 mol/L升高到 0.3 mol/L和1 mol/L时,盐桥E14-R18,K19-E74,K25-E26,E140-K147和E140-R151都消失了,这些盐桥也受到溶液温度升高的影响[13],表明上述盐桥可能对溶液温度和NaCl浓度都比较敏感,这应在今后相关研究中着重考察的指标之一。"
| [1] 朱玉珍,张庆文.神经元钙传感蛋白研究前沿与热点分析[J].中国组织工程研究,2014,18(42):6856-6862.[2] 朱玉珍,邹昱,张庆文.将分子动力学模拟方法引入体育领域研究的可行性分析--以肌动蛋白、肌球蛋白和人类神经元钙传感蛋白为例[J],体育科学,2017,37(1):90-97.[3] 朱玉珍,张庆文.分子动力学模拟:神经元钙传感蛋白生理机制研究的新方向[J].中国组织工程研究,2017,21(20):3224-3233.[4] Drumond LE, Mourao FA, Leite HR, et al. Differential effects of swimming training on neuronal calcium sensor-1 expression in rat hippocampus/cortex and in object recognition memory tasks. Brain Res bulletin.2012;88:385-391.[5] White LJ, Castellano V. Exercise and brain health--implications for multiple sclerosis: Part 1-neuronal growth factors.Sports medicine. 2008;38: 91-100.[6] Saab BJ,Georgiou J,Nath A,et al.NCS-1 in the dentate gyrus promotes exploration, synaptic plasticity, and rapid acquisition of spatial memory. Neuron.2009;63: 643-656.[7] Dason JS,Romero-Pozuelo J,Atwood HL, et al. Multiple roles for frequenin/NCS-1 in synaptic function and development.Molecular neurobiology.2012;45: 388-402.[8] Ardoy DN,Fernandez-Rodriguez JM,Jimenez-Pavon D,et al.A Physical Education trial improves adolescents' cognitive performance and academic achievement:the EDUFIT study. Scand J Med Sci Sports. 2014;24(1):e52-61[9] Pongs O,Lindemeier J,Zhu XR,et al.Frequenin--a novel calcium-binding protein that modulates synaptic efficacy in the Drosophila nervous system.Neuron.1993;11: 15-28.[10] Hilfiker S.Neuronal calcium sensor-1: a multifunctional regulator of secretion.Biochem Soc Trans. 2003;31:828-832.[11] Burgoyne RD.Neuronal calcium sensor proteins: generating diversity in neuronal Ca2+ signalling. Nat Rev Neurosci.2007;8: 182-193.[12] Martin VM,Johnson JR,Haynes LP,et al.Identification of key structural elements for neuronal calcium sensor-1 function in the regulation of the temperature-dependency of locomotion in C. elegans.Mol Brain.2013;6: 39.[13] Zhu Y, Ma B, Qi R, Nussinov R, et al.Temperature-Dependent Conformational Properties of Human Neuronal Calcium Sensor-1 Protein Revealed by All-Atom Simulations.J Phys Chem B.2016; 120:3551-3559.[14] 郭浙斌.低氧下运动及补液对集体体液平衡和有氧运动能力的影响[D].华南师范大学,2007.[15] Heidarsson PO,Bjerrum-Bohr IJ,Jensen GA,et al.The C-terminal tail of human neuronal calcium sensor 1 regulates the conformational stability of the Ca 2+- activated state.J Mol Biol. 2012;417: 51-64.[16] Bourne Y,Dannenberg J,Pollmann V,et al.Immunocytochemical localization and crystal structure of human frequenin (neuronal calcium sensor 1).J Biol Chem.2001;276: 11949-11955.[17] Zhu Y, Wu Y, Luo Y, et al.R102Q mutation shifts the salt-bridge network and reduces the structural flexibility of human neuronal calcium sensor-1 protein.J Phys Chem B.2014;118: 13112-13122.[18] 朱玉珍,张庆文.神经元钙传感蛋白R102Q 突变体的动力学构象特征[J].中国组织工程研究,2015,19(2):225-230.[19] 朱玉珍.神经元钙传感蛋白构象动力学及调控机制研究[D].上海体育学院.2016.[20] Gifford JL,Walsh MP, Vogel, HJ.Structures and metal-ion-binding properties of the Ca2+-binding helix-loop-helix EF-hand motifs. Biochem J.2007;405: 199-221.[21] Hess B, Kutzner C, van der Spoel D,et al.GROMACS 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation.J Chem Theory Comput.2008;4: 435-447.[22] Bjelkmar P, Larsson P, Cuendet MA, et al.Implementation of the CHARMM force field in GROMACS: analysis of protein stability effects from correction maps, virtual interaction sites, and water models.J Chem Theory Comput.2010;6:459-466.[23] Bussi G,Donadio D,Parrinello M.Canonical sampling through velocity rescaling.J Chem Phys. 2007;126: 014101.[24] Parrinello M, Rahman A. Polymotphic transitions in single-crystals-a new molecular-dynamics method.J Appl Phys.1981;52: 7182-7190.[25] Berk Hess,Hemk Bekker,Herman J.C.Berendsen,et al. LINCS: A linear constraint solver for molecular simulations.J Comput Chem.1997;18: 1463-1472.[26] Shuichi Miyamoto,Peter A.Kollman.Settle- an analytical version of the shake and rattle algorithm for rigid water models.J Comput Chem.1992;13: 952-962.[27] Kabsch W, Sander C.Dictionary of potein secondary structure - pattern -recognition of hydrogen -bonded and geometrical features. Biopolymers.1983;22:2577-2637.[28] Humphrey W,Dalke A,Schulten K.VMD: visual molecular dynamics. Journal of molecular graphics. 1996;14:33-38,27-28.[29] Eargle J, Luthey-Schulten Z.NetworkView: 3D display and analysis of protein.RNA interaction networks.Bioinformatics. 2012;28: 3000-3001.[30] Zhu Y, Yang S, Qi R, et al.Effects of the C-Terminal Tail on the Conformational Dynamics of Human Neuronal Calcium Sensor-1 Protein.J Phys Chem B.2015;119:14236-14244.[31] Sethi A,Eargle J,Black AA,et al.Dynamical networks in tRNA:protein complexes. Proc Natl Acad Sci U S A. 2009;106(16):6620-6625.[32] Girvan M,Newman ME.Community structure in social and biological networks. Proc Natl Acad Sci U S A. 2002;99(12): 7821-7826[33] Strahl T,Huttner IG,Lusin JD,et al.Structural insights into activation of phosphatidylinositol 4-kinase (Pik1) by yeast frequenin (Frq1).J Biol Chem.2007;282(42):30949-30959.[34] Hendricks KB,Wang BQ,Schnieders EA,et al.Yeast homologue of neuronal frequenin is a regulator of phosphatidylinositol-4-OH kinase.Nat Cell Biol.1999;1(4):234-241.[35] Kabbani N,Negyessy L,Lin R,et al.Interaction with neuronal calcium sensor NCS-1 mol/Lediates desensitization of the D2 dopamine receptor. J Neurosci. 2002 Oct 1;22(19):8476-8486.[36] Kumar S, Ma B, Tsai CJ, et al.Folding funnels and binding mechanisms.Protein eng.1999;12 (9):713-720.[37] Waldburger CD, Schildbach JF, Sauer RT. Are buried salt bridges important for protein stability and conformational specificity? Nat Struct Biol.1995;2: 122-128.[38] Tissot AC, Vuilleumier S, Fersht AR. Importance of two buried salt bridges in the stability and folding pathway of barnase. Biochemistry.1996;35: 6786-6794.[39] Makhatadze GI,Loladze VV,Ermolenko DN,et al.Contribution of surface salt bridges to protein stability: guidelines for protein engineering.J Mol Biol.2003;327: 1135-1148.[40] Ibarra-Molero B,Zitzewitz JA, Matthews CR.Salt-bridges can stabilize but do not accelerate the folding of the homodimeric coiled-coil peptide GCN4-p1.J Mol Biol.2004;336: 989-996.[41] Bellucci L,Corni S,Di Felice R,et al.The structure of neuronal calcium sensor-1 in solution revealed by molecular dynamics simulations.PLoS One.2013;8: e74383. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Liu Zhichao, Zhang Fan, Sun Qi, Kang Xiaole, Yuan Qiaomei, Liu Genzhe, Chen Jiang. Morphology and activity of human nucleus pulposus cells under different hydrostatic pressures [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1172-1176. |
| [3] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [4] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [5] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [6] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [7] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [8] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [9] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [10] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [11] | Xing Hao, Zhang Yonghong, Wang Dong. Advantages and disadvantages of repairing large-segment bone defect [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 426-430. |
| [12] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| [13] | Wang Hao, Chen Mingxue, Li Junkang, Luo Xujiang, Peng Liqing, Li Huo, Huang Bo, Tian Guangzhao, Liu Shuyun, Sui Xiang, Huang Jingxiang, Guo Quanyi, Lu Xiaobo. Decellularized porcine skin matrix for tissue-engineered meniscus scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3473-3478. |
| [14] | Mo Jianling, He Shaoru, Feng Bowen, Jian Minqiao, Zhang Xiaohui, Liu Caisheng, Liang Yijing, Liu Yumei, Chen Liang, Zhou Haiyu, Liu Yanhui. Forming prevascularized cell sheets and the expression of angiogenesis-related factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3479-3486. |
| [15] | Liu Chang, Li Datong, Liu Yuan, Kong Lingbo, Guo Rui, Yang Lixue, Hao Dingjun, He Baorong. Poor efficacy after vertebral augmentation surgery of acute symptomatic thoracolumbar osteoporotic compression fracture: relationship with bone cement, bone mineral density, and adjacent fractures [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3510-3516. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||