Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (22): 3567-3575.doi: 10.3969/j.issn.2095-4344.2017.22.021
Previous Articles Next Articles
A review on nano-materials as joint replacement implants
- 1Department of Orthopedics, the 4th Affiliated Hospital of China Medical University, Shenyang 110032, Liaoning Province, China; 2Department of Rehabilitation, Shengjing Hospital of China Medical University, Shenyang 110134, Liaoning Province, China; 3Department of Orthopedics, the 1st Affiliated Hospital of China Medical University, Shenyang 110000, Liaoning Province, China
-
Online:2017-08-08Published:2017-09-01 -
Contact:Zhang Hang-zhou, M.D., Associate chief physician, Department of Orthopedics, the 1st Affiliated Hospital of China Medical University, Shenyang 110000, Liaoning Province, China -
About author:Wang Lin, Master, Physician, Department of Orthopedics, the 4th Affiliated Hospital of China Medical University, Shenyang 110032, Liaoning Province, China -
Supported by:the National Natural Science Foundation of China, No. 81671811, 81501857
CLC Number:
Cite this article
Wang Lin, Chen Dan-ying, Zhang Zhi-yu, Zhang Hang-zhou.
share this article
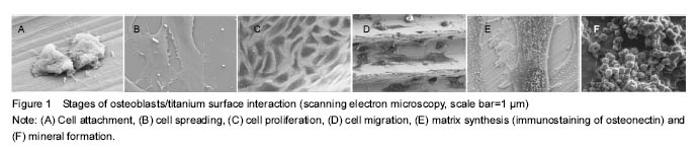
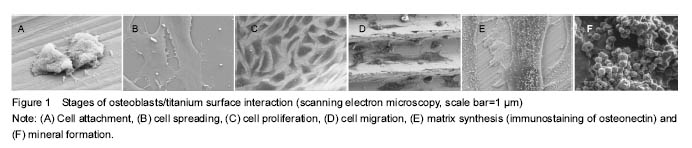
Basic profile In these 51 articles related to the study, there are 15 articles addressing the bio-response to nano-materials; 4 articles addressing biocompatibility issues of nano-materials; 11 articles concerning bacteria and nano-materials; 28 articles focusing on improving biocompatibility of nano-materials in implants. Bio-responses to nano-materials With the advancement of nanotechnology and its application in joint surgery, biocompatibility is a stringent requirement for implnats. This requirement cannot be achieved without knowledge of responses of cells to biomaterial interfaces with cells. Cells are said to be sensitive to biochemical, mechanical and topological cues[7]. The focus here is how osteoblasts, the most crucial bone cells, respond to biomaterials at micro- and nano-structural levels. When a biomaterial is implanted into a joint, a bio-response in the process of wound healing occurs. This bone healing starts with protein adsorption unto the surface which is influenced by physicochemical properties of the material. This is followed by the attachment phase which occurs more rapidly. Then finally, the adhesion phase occurs over a long period, involving various biomolecules and also influenced by the biophysical environment of the interface (Figure 1)[8]. This response can either lead to osseointegration of the implant or failure of the implant. This osseointegration is determined by osteoblasts and other proteins around the interface. Outcomes may have extensive implications on the success of the implant and the host as well. These surface properties primarily determine the interaction between the materials and proteins in the biological environment as well as interactions with the cells tissues[9]. Several researches have been done to evaluate cellular responses to various nano materials with various surface properties. Some of the results are heterogeneous since in vivo and in vitro conditions are different and also because of the different materials and methods used[10-13]. In one experiment conducted by Meireles et al[10] to evaluate the effect of hydroxyapatite and titanium nanostructures on early in vivo bone response, they found out that there was an increase in bone contact for titanium coated implant compared to hydroxyapatite coated implant. This and many others have confirmed the assumption that osteoblasts respond differently to different surface properties of same or different materials. The responses expected on a bone/material interface are inflammation and thrombosis[9]. The inflammatory responses can range from mild to severe or chronic. Chronic inflammation can result in thick fibrous capsule which can lead to an undesirable damage to surrounding tissue and failure of the implant. This undesirable effect is due to the persistent white cells around the implant causing the inflammatory response. Small amounts of metallic ions, such as nickel, chromium, molybdenum, cobalt, and, to a lesser extent, tantalum and titanium, are said to be recognized as antigens when bounding with native proteins to form hapten complexes. These complexes can then activate the immune system and generate a metal hypersensitivity, mostly through a type IV delayed cell-mediated response leading to inflammation[14]. Bengt Fadel in his review of clear and danger of engineered nanoparticles stated that nanoparticles, due to their hydrophobicity, may cause an immune response since hydrophobic portions of biological molecules acts as signals to initiate immune responses. Nanoparticles, according to Reisetter et al [15], can attract macrophages and make the macrophages to secret pro-inflammatory cytokines when picked up, which can lead to cell death in some cases. Their data showed that, after phagocytosis of carbon black nanoparticles, macrophages increased in size, which is the opposite of normal programmed cell death. This increase in size led to inflammasome activation and ultimately death of the cells. The cell death subsequently caused release of cellular contents that might further be harmful by causing more immune responses[16]. There have also been reports of interactions between nano-materials and the complement systems. This interaction is also influenced by several factors of the material including size, morphology and surface characteristics[17]. One of the limitations of prosthetic device is its ability to form thrombus when it comes in contact with blood cells[18-19]. But studies have shown that some prosthetic materials have thrombo-resistance property while others do not. These thrombi apart from the risk of emboli formation can serve as a locus for bacteria colonization, which might lead to failure of the prosthesis in severe cases. Evidence has also shown the effect of differences in mechanical and physical chemical environments of cells on cell growth, apoptosis and differentiation[7]. One study by Dike and colleagues[20], showed that endothelial cells within 72 hours of culture on thin line of fibronectin shut off both growth and apoptosis programs and underwent differentiation, resulting in the formation of capillary tube-like structures containing a central lumen. Cells cultured on wider (30 μm) lines also formed cell-cell contacts and aligned their actin cytoskeleton, but these cells spread to larger areas (2 200 μm2), proliferated, and did not form tubes. Use of micropatterned substrates reveals that altering the geometry of cell spreading can switch endothelial cells among the three major genetic programs that govern angiogenesis-growth, apoptosis and differentiation[20]. More experiments are warranted to see this effect in other cells like osteoblasts on nanometer scale substrates. Understanding the various responses of cells to biomaterials will help in the production of modern implants that are of high standards, which can better fit in the physiological environments that they are used, with less or no complications. Biocompatibility of nano-materials Even though nano-materials have made debuts in the medical and biomedical world, there are still lots of concerns regarding their use in implants. Safety issues regarding cytotoxicity among others are being raised. No matter the duration of use, these implants must meet certain requirements in order to be compatible with surrounding tissue and the body as a whole. Some of these requirements according to the ISO 10993 standards include non-toxicity, non-thrombogenicity, non- carcinogenic, non-antigenic and non-mutagenic[9]. Unfortunately, no implants have been developed so far that can meet all of the above standards, as well as allow full, unrestricted and high impact activity like in a normal healthy joint, which can last the entire life of the patient. Many studies have shown tremendous undesirable effects of nano-materials on cells[21-24]. This has prone more research in nanotechnology and nanomedicine in order to counter these undesirable effects while improving on the biocompatibility for implants in the future. More of concerns especially for implants that are placed for long term or permanent use are biostability and infection. Biostability refers to the ability of a material to resist biodegradation mechanisms by the body and be able to maintain its properties in situ. This means that any biomaterial must not be released nor be degraded by any means such as hydrolysis, oxidation, enzyme catalyzed enhancement of hydrolysis, oxidation, lipid absorption, swelling and calcification[9]. Materials chosen for orthopedic implants must possess these qualities in order to enhance biocompatibility. Most of the materials used in traditional implants are biodegradable. Recent advances in nanotechnology have turned to the combination of bioactive materials in order to improve biocompatibility of implants. This, however, according to the study of Helmus and colleagues, has it own effects due to the methods used in combining these materials[9]. The properties of these materials can be affected, which can lead to reduced strength of the materials and ultimately affects biostability and biocompatibility. A biologically stable implant is definitely bound to reduce many undesirable bio-responses as well as toxic effects on the normal cells, especially osteoblasts. Bacteria and nano-materials One of the many serious complications encountered in joints surgery is bacterial infection of the implants. Despite proper aseptic techniques and sterilization methods, the incidence of bacterial infections still remain a challenge in medical practice, and it is one of the main reasons for implant failure and revision surgeries in joint replacements. Reports have shown that about 4.3% of all orthopedic implants done in the USA become infected annually[25]. Even with antibiotic prophylaxis, implant related infections still remain high. This has increased the burden on patients, as it is not only costly, but it prolongs recovery time and even sometimes leads to amputation in severe cases. What then could be responsible for this complication despite all necessary measures taken? The host’s immunity is one important factor that helps fight infection. With a compromised immune status the risk of infection increases significantly. Studies have also shown that the route of administration of the usual antibacterial agents is not sufficient enough to fight bacteria especially those that colonize implants during surgery. Some anti-bacterial agents have poor penetrability into bone tissues where these colonizations occur around the implants. In recent years, there are many strains of bacteria that are becoming resistant to traditional anti-microbial agents. These and many other factors have been the major reasons for bacterial infections of implants. But one important phenomenon which has been of interest to many researchers is the formation of biofilms which are more resistant to any anti-microbial agent. A biofilm is a group of organisms that adhere to a surface of any material and are protected by an extracellular hydrophilic polymeric substance they produce, the glycocalyx[26]. Biofilms are said to consist of stacks of bacteria with aqueous channels flowing in between, which makes impenetrability very unlikely[27-28]. These bacteria also produce antibiotic-inactivation enzymes which accumulate within the gycocalyx to protect underlying cells[29]. This protective mechanism by the biofilm makes it resistant to any known anti-microbial agent. It is postulated that, there is a competition between osseointegration of an implant and bacterial adhesion to the material surface just after implantation. This means that if bacterial adhesion occurs before osseointegration and if the bacteria overcome the defensive mechanisms of the body, colonization is bound to occur and failure of that implant is possible especially if the colonization leads to biofilm formation. Antibiotic therapy during an implant infection only resolves symptoms caused by floating single planktonic bacteria shed from biofilms[30]. Studies have shown that the type of materials used in some prostheses make them susceptible to bacterial adhesion[25]. According to one study, the physicochemical properties of a material is said to mediate bacteria adhesion. Bacterial adhesion, they said, is preceded by surface adhesion of small molecules including proteins which occur as a result of interactions between the bacteria and surface of the substrate. The reduction of particle size in implants to the nanoscale, according to numerous researches, has helped reduce bacteria colonization and improve osseointegration[31-33]. As the size of materials is reduced to the nanoscale base on nanotechnology, the resulting properties of the material change noticeably[31]. Azam et al[31], in an experiment to see the effect of nanoparticles on bacteria, assumed that the properties of nanometer sized materials change with size, they used different sizes nanoparticles of CuO which were produced by gel-combustion method. They cultured E. coli, P. aeruginosa (Gram negative bacteria) and B. subtilis, S. aureus (Gram positive bacteria) and seeded them onto agar plates. Solution of CuO nanoparticles of different sizes were then poured into wells punched on the agar plates containing bacteria and incubated for 24 hours. The minimum inhibitory concentration and minimum bactericidal concentrations were then determined. They found that, the smallest size nanoparticles showed significant inhibitory effect on both Gram positive and Gram negative bacteria as compared to those of larger sizes. As a control, they used tetracycline, and they noticed also that, the zone of inhibition for the nanoparticles were either equal to or greater than that of tetracycline. This was even more prominent for the B. subtilis strain. They concluded that the release of ions due to interactions between particle layer and solution caused bactericidal activity which caused alterations in the bacteria membranes. It was also noted that sensitivity or resistance of bacteria to nanoparticles varies for both Gram positive and Gram negative bacteria. This could be due to the differences in the cell structures, physiology, metabolism or degree of contact with particles. Gram positive bacteria are said to have greater affinity for copper than gram negative bacteria. From their experiment we can conclude that nano-materials can act as anti-microbial agents, and that their activity is size dependent. Bacteria are said to be in micron size range with nanometer range of pores in their membranes. This makes nano-particles to cross cell membranes of bacteria and cause alterations in their membranes. Similar experiment was also done by Tsao et al[31], where they suggested that the exposure of gram positive bacteria to caboxyfullerene nanoparticles resulted in puncturing of the bacteria leading to cell death[32]. However, their study did not show any effect of these nanoparticles on normal cells like osteoblasts. This would make some wonder whether these nanoparticles will show a similar effect with normal cells. The surface properties of nano-materials vary not only with size of particles but also with the technique used to produce the material as well as the surface treatment technique used. According to Ercan et al[33], fighting bacterial infection in implants should be geared towards preventing bacterial adhesion and promoting bacterial death at the time of implantation before the formation of biofilm. This can be achieved by surface treatment of nanoparticles. In their experiment, various sizes of nanoparticles were synthesized through anodization technique to produce nanotubes of 20, 40, 60 and 80 nm from pure titanium foil. Some conventional titanium (cleaned pure titanium) was heat treated as well. Surface roughness and surface chemistry of the tubes were analyzed by different methods. Phase and contact angle analysis were also done. Bacterial culture of Staphylococcus epidermis and S. aureus was done and seeded on tubes and allowed to adhere. After an incubation period of 1 hour, tubes were rinsed and inserted in glycerol and Nacl solution prior to imaging. Visualization and counting of live and dead bacteria were done with fluorescence microscope. They found out that the nanotubes had greater nano roughness. Florine that is known to be water soluble was also found on the surfaces of the nanotubes. Fewer bacteria density was observed on nanotubes titanium as well as on the heat treated conventional titanium. Fewer living bacteria were also observed on the larger nanotubes titanium. The authors therefore concluded that, there was a change in surface chemistry after surface nanomodification by anodization and heat treatment of the titanium. This led to a significant change in bacterial responses. They found out that heat treatment was able to remove fluorine on anodized titanium which may have impacted the antibacterial properties of the surface. The most robust anti-bacterial effect was seen in the 80 nm anodization synthesized nano-Ti which had decreased adhesion for both live and dead bacteria. In a similar in vitro study, conducted by Puckett and colleagues[34], to investigate the relationship between nanostructure of titanium surfaces and bacterial attachment, researchers examined the adhesion of Staphylococcus aureus, Staphylococcus epidermidis, and Pseudomonas aeruginosa on conventional titanium, nanorough titanium produced by electron beam evaporation, and nanotubular and nanotextured titanium produced by two different anodization processes. Their study found that compared to conventional (nano-smooth) titanium, the nanorough titanium surfaces produced by electron beam evaporation decreased the adherence of all of the aforementioned bacteria the most, while the anodized nanotubes had more adherent bacteria. The conventional and nanorough titanium surfaces were found to have crystalline TiO2, while the nanotubular and nanotextured titanium surfaces were found to be amorphous. The surface chemistries were similar for the conventional and nanorough titanium, while the anodized titanium surfaces contained fluorine. The study demonstrated that certain nanometer sized titanium topographies may be useful for reducing bacteria adhesion but promoting bone tissue formation and, thus, should be further studied for improving the efficacy of titanium-based orthopedic implants. Their experimental findings are in line with those of Ercan and colleagues. Puckett et al[34]. did not heat treat the anodized titanium nanotubes, and therefore the flourine remained trapped in the nanotubes. These trapped flourine is hypothesized by the authors to have affinity for bacteria. Since other works have demonstrated increased osteoblast functionality with titanium nano-materials[35], observations made in these experiments make us conclude that controlled anodized titanium nanotube formation and treatment could serve as strong candidates for the design of future implant materials with improved antimicrobial behaviors. These effects can be seen to reduce bacterial adhesion, thereby preventing colonization and biofilm formation. However, these studies have to be done in vivo to compare their effects and biocompatibility. Improving biocompatibility of nano-materials in implant preparation As already discussed above, any implant must meet certain requirements. An implant must be biologically stable, able to promote osseointegration, and able to prevent bacteria adhesion and growth. A biologically stable implant is not supposed to be toxic, thrombogenic, carcinogenic, antigenic and mutagenic. However, there is hardly any material that has met all these criteria even with the advancement and application of nanotechnology. Biocompatibility of implants depends on many factors, which include but not limited to the type of material used, size of the nanoparticles of the material, methods used in the preparation of the materials, surface treatment techniques and conditions under which the materials are bproduced or treated, as well as factors of the patient or the host’s environment. For improved biocompatibility and high functionality of implants, these factors need to be taken into consideration. Many nano-materials have shown to promote osteoblast proliferation in vitro [34-36], while some are said to be cytotoxic[24, 37-39]. One material with such high biocompatibility is titanium. As seen in the experiments of Puckett and colleagues and many others[34, 40]. Titanium and its alloys have been extensively used as biomaterials in bone surgeries in recent decades because of their generally good biocompatibility which is mainly due to its mechanical properties that make it better adapted to those of bone, and because its surface is always covered by passive nanometer layer making it corrosion resistance and biologically inert in vivo [36]. Recent advances in nanotechnology have made it possible to manufacture nano structured materials that have been extensively used in modern implants. Nano structured materials are said to have many properties which include large surface area, high surface energy, high spatial confinement and reduced imperfections[7]. These properties depend on the size of the nanoparticles of the material. Studies have shown that, as the size of nanoparticles is reduced, cellular and material interaction is enhanced[31-33]. This reduction in size to few nanometers is achieved by the fabrication method used. Some methods also lead to more biostable material than others. Titanium nanotubes have been prepared by methods such as sol-gel method, electrophoretic deposition and anodization methods. Details of the fabrication techniques can be reviewed elsewhere. However, many prefer to use the anodization method due to its strong adherent property of titanium oxide layer which has yielded good effects. In an experiment by Oh and colleagues[41], titanium oxide nanotube was synthesized by anodization. The nanotubes were then treated with NaOH to make it bioactive. This was followed by heat treatment to make them into an anatase structure. Bone growth was evaluated on the surfaces of the nanotubes by soaking the tubes in simulated body fluid that contained ion concentrations nearly equal to that of the human blood plasma. Microstructural/morphological evaluation was done using scanning electron microscope. It was noted by the authors that various nano-sizes of TiO2 were synthesized at a very short anodizing time of about 1 minute. At longer anodizing time various geometry of TiO2 nanotubes with various inner, outer diameters and wall thicknesses, were produced. They also established that, the anatase phase TiO2 is much more efficient in nucleation and growth of hydroxyapatite. They attributed the hydroxyapatite formation to interactions of several factors on the surface of implant materials in the presence of simulated body fluid such as surface area, surface roughness, electrical charge of the host substrate, concentration and pH of the simulated body fluid. Hydroxyapatite is said to be formed easily in the presence of simulated body fluid[42]. They concluded from that study that bioactive nanotubes can be produced when treated with NaOH after anodization, and can be used as bioactive surface layers on titanium implant metals and alloys. Another common method that has been widely used to enhance biocompatibility of implants material is surface treatment or modification. For long-term and permanent implants, long-term or permanent stability is required which can be achieved by surface modification, for a faster and better osseointegration of the implant. A better biocompatible surface must be able to prevent unwanted adhesion, attract adhesion of specific, wanted cells and promote proliferation of that cell. Many surface treatment methods have been used in several researches. Some are just simple physical or chemical treatments[43-44], while others involved a mixture of both to create a layer of coating on the surface of the material. One widely used method is the coating of surfaces with hydroxyapatite, a form of apatite, which is used to mimic the bone structure. Hydroxyapatite constitutes approximately 70% of the total bone mass, while the extracellular bone constitutes 20% of it[45]. Many studies have reported enhanced osteoblast interaction with hydroxyapatite on coated nano surfaces and thereby enhancing osseointegration[10-11, 46-48]. This enhanced effect according to Meirelles et al[10] may be due to the bioactivity or chemistry of hydroxyapatite and or the topography of the implanted nano-size structure. In one randomized controlled trial conducted to determine if pin related complications can be reduced by the use of hydroxyapatite coated pins in fixators used for distracting osteogenesis[46], 46 patients undergoing segmental transport or tibia lengthening were randomized into two groups. The control group received uncoated stainless titanium Schanz pins, while the study group received hydroxyapatite coated stainless-steel Schanz pins. Both types of pins were of similar dimensions. The patients were then monitored for loosening and infection for 38 weeks. At the end of treatment, the pins were removed and measured for stability of the pin-bone bonding. The extraction torque of the pins was determined by means of an electronic torque wrench. No loosening or infection was observed in the study group, while 13% and 90% of pins in the control group had loosening and infection respectively, with mean extraction torque of (0.43±0.18) N-m and (0.10±0.09) N-m in the study and control groups respectively. The authors found out that. Pin loosening and infection occurred as early as 4 weeks in the control group. They concluded that hydroxyapatite coatings of the pins reduce loosening and infection associated with Schanz pins. Even though the result obtained from their study was excellent but a flaw was observed, that is the use of two different materials of pins with different material properties and strength, makes one doubt the comparability of the pins. Many novel experiments have been performed on the wonders of hydroxyapatite coated materials[47-48]. Hydroxyapatite coatings have been regarded as one of the best ways to improve biocompatibility of implant materials as it provides a favorable surface for both osteoblasts and fibroblasts activity. But Antonov and colleagues[49] in their study said that the enhancement of cell bioactivity is dependent on the stability of the coatings with respect to erosion. In their experiment wherein hydroxyapatite was deposited on both polymer (teflon and polyethylene) and alloy (titanium) surfaces by two different methods (KrF laser and CO2 laser), they found out that hydroxyapatite coatings deposited on teflon and polyethylene surfaces dissolved at different rates after contact with simulated body fluid. Only about 10% of the initial coating on polyethylene remained after 6 hours of contact with simulated body fluid. MTT test also revealed apparent cytotoxicity of hydroxyapatite coated polyethylene which led to poor cell growth. One of their most significant finding was that the hydroxyapatite coating on titanium alloy suppressed fibrous tissue formation which was found in vivo. Although no significant osteogenesis was observed among KrF laser deposited coatings, CO2 laser deposited coatings had a markedly higher osteointegration rate than the KrF laser deposited coatings. It can be concluded from their study that the bioactivity of hydroxyapatite is determined by its physicochemical properties which are controlled by substrate and coating parameters. From their experiment we can also see that hydroxyapatite-titanium material promotes osseointegration but reduces fibrosis of surrounding tissues. In another study by Meirelles and colleagues[10], the current view of enhanced bone formation or osseointegration of hydroxyapatite was not supported. They used two groups of implants with tatania or hydroxyapatite coatings. Tatania coating was done by sol-gel technique while hydroxyapatite was prepared by mixing H3PO4 and Ca(NO3)2 with a Ca/P molar ratio of 1.67 in the presence of a liquid crystalline phase. The coatings were then applied to implants by dip coating technique. Topographic analysis was done before implanting into the rabbit tibia. After 5 weeks, the rabbits were killed and histologic evaluation was done. Although the result was not statistically significant, there was a 24% increase in bone contact for titania compared to hydroxyapatite measured on the lateral wall of implants. Also there was 170% increase in the apical wall for titania compared to 19% for hydroxyapatite, even though the apical parts of titania were in the bone marrow. The authors were concerned about the origin of the cells since the apical parts were not in direct contacts with the cortical bone but in the bone marrow. Scholars wonder whether they could have been from the bone marrow stem cells or from the opposing cortex. However they believed that the low bone formation on the lateral walls close to the implant’s apical part might indicate that cells were preferentially recruited from the opposing cortex. Since all the implants were in contact with the bone marrow, we believe that these cells might have come from stem cells in the bone marrow. Another factor that might be responsible for such tremendous apical bone growth could have been due to the interactions of the implant with the plasma fluid of the bone marrow as was seen in the experiment by Oh et al.[41]. However, the authors concluded that the difference in bone formation on the implants was as a result of surface nano roughness of the implants. Nano-titania implants, due to increase surface roughness exhibit increased feature density and larger feature coverage area compared to nano-hydroxyapatite. This, they said, may represent more binding sites for protein cell attachment. The beneficial chemical effect of hydroxyapatite as shown in many studies was of little relevance in their model. One novel way thought of enhancing biocompatibility of implants is the loading of antibiotics into these nano-materials to prevent bacteria adhesion. This is believed to prevent or reduce implants infection. Since antibiotics administered systemically are poorly delivered to the implants where they are needed, delivering them locally in the implants will be the best option in curbing implants associated infections which have been responsible for failure in most implant surgeries. However, many factors are to be considered when thinking of this mode of drug delivery. Since the main goal after implant surgery is enhanced osseointegration of the implant as well as reduced or no bacteria adhesion, the drug used must have no effect on necessary proteins and osteoblasts, they must be effective against both gram positive and negative bacteria, released and effective for a long period of time, not destroyed by loading method, safe for any group of patients, etc. One medium that has been extensively tried in experiments to deliver antibiotics is hydroxyapatite, even though other media have been used[25, 35, 50-51]. To counter the disadvantages of some of the drug delivery systems, Shinto and colleagues[50] placed three kinds of antibiotics powder (gentamicin, cefoperazone and flomoxef) in calcium hydroxyapatite ceramic blocks with cavities. In the in vitro experiment, they placed 75 mg of each antibiotic into six large blocks and placed each block in a tube containing phosphate buffer saline (PBS). PBS was replaced at an interval of 24 hours, antibiotics concentration of the eluted fluids were later determined. Bactericidal activity of the eluted fluids of gentamicin was determined by placing 0.1 mL of sample on a trypticase soy agar plate containing S. aureus and cultured for 24 hours. Zones of inhibition were later recorded and compared. In the in vivo experiment, small blocks each containing 5 mg of gentamicin, were implanted into proximal tibia of rabbits. After regular intervals, specimens of local bones, muscle, plasma, kidneys and liver were taken after killing the rabbits and antibiotics assay done on them. Gentamicin blocks were placed into tibia of seven rabbits, and these rabbits were killed at monthly intervals. Specimens were then taken from tibia and stained for histology. The authors found out that, gentamicin release in both in vitro and in vivo experiments were similar, though gentamicin peaking occurred later (8 days) in the in vivo experiment as compared to 2 days in vitro. Gentamicin release lasted for about 90 days. The other drugs showed similar trend but peaked earlier and lasted for a short time. Gentamicin concentrations in the other tissues remained low compared to that of the bones. In vitro fluids showed strong inhibitory effects for a long time meaning that, they were chemically stable and fully active. They concluded that biocompatibility was not affected by gentamicin. Similar experiment done by Krisanapiboon and colleagues[51], using gentamicin, fosfomycin, imipenem and amphotericin B, found out that the antibiotics had no effect on osteoblast morphology when incubated for 48 hours, but the percentage of viable cells decreased at higher concentrations except for imipenem. Imipenem at the highest used concentration had a significantly higher percentage of viable cells than at lower concentration. The percentage of viable cells for gentamicin decreased only slightly at the highest concentration used which was not significantly too different from the control. Thus, we can see that gentamicin loaded in hydroxyapatite can be a good medium to deliver drug locally. But due to its limitation, especially in the covering of gram-positive bacteria and resistance of some bacteria, it cannot be a very good agent for implants. More research involving broad spectrum antibiotics has to be carried out in order to tackle the issue of implant-related infections. "
| [1] Crowninshield RD, Rosenberg AG, Sporer SM. Changing demographics of patients with total joint replacement. Clin Orthop Relat Res. 2006;443:266-272. [2] Huang SL, He XJ, Wang KZ. Joint replacement in China: progress and challenges. Rheumatology (Oxford). 2012;51(9):1525-1526. [3] Jin SE, Jin WB, Hong S. Multi-scale Observation of biological interations of Nanocarriers from Nano to Macro. Microsc Res Tech. 2010;73(9):813-823. [4] Kam LC. Capturing the nanoscale complexity of cellular membranes in supported lipid bilayers. J Struct Biol. 2009;168(1): 3-10. [5] Zolnik BS, González-Fernández A, Sadrieh N, et al. Nanoparticles and the immune system. Endocrinology. 2010;151(2):458-465. [6] Tributsch H, Copf F, Copf P, et al. Nano-material aspects of shock absorption in bone joints. Open Biomed Eng J. 2010;4:257-262. [7] Qian T, Wang Y. Micro/nano-fabrication technologies for cell biology. Med Biol Eng Comput. 2010;48(10):1023-1032. [8] Meyer U, Büchter A, Wiesmann HP, et al. Basic reactions of osteoblasts on structured material surfaces. Eur Cell Mater. 2005;9:39-49. [9] Helmus MN, Gibbons DF, Cebon D. Biocompatibility: meeting a key functional requirement of next-generation medical devices. Toxicol Pathol. 2008;36(1):70-80. [10] Meirelles L, Melin L, Peltola T, et al. Effect of hydroxyapatite and titania nanostructures on early in vivo bone response. Clin Implant Dent Relat Res. 2008;10(4):245-254. [11] Meirelles L, Arvidsson A, Andersson M, et al. Nano hydroxyapatite structures influence early bone formation. J Biomed Mater Res A. 2008;87(2):299-307. [12] Meirelles L, Currie F, Jacobsson M, et al. The effect of chemical and nanotopographical modifications on the early stages of osseointegration. Int J Oral Maxillofac Implants. 2008;23(4): 641-647. [13] Traini T, Murmura G, Piattelli M, et al. Effect of nanoscale topography of titanium implants on bone vessel network, osteocytes, and mineral densities. J Periodontol. 2013;84(10): e40-e47. [14] Thierry B, Tabrizian M. Biocompatibility and biostability of metallic endovascular implants: state of the art and perspectives. J Endovasc Ther. 2003;10:807-824. [15] Reisetter AC, Stebounova LV, Baltrusaitis J, et al. Induction of inflammasome-dependent pyroptosis by carbon black nanoparticles. J Biol Chem. 2011;286(24):21844-21852. [16] Moghimi SM, Andersen AJ, Ahmadvand D, et al. Material properties in complement activation. Adv Drug Deliv Rev. 2011;63(12):1000-1007. [17] Salvador-Morales C, Flahaut E, Sim E, et al. Complement activation and protein adsorption by carbon nanotubes. Mol Immunol. 2006;43(3):193-201. [18] Varu VN, Tsihlis ND, Kibbe MR. Basic science review: nitric oxide--releasing prosthetic materials. Vasc Endovascular Surg. 2009;43(2):121-131. [19] Jordan SW, Chaikof EL. Novel thromboresistant materials. J Vasc Surg. 2007;45:104-115. [20] Dike LE, Chen CS, Mrksich M, et al. Geometric control of switching between growth, apoptosis, and differentiation during angiogenesis using micropatterned substrates. In Vitro Cell Dev Biol Anim. 1999;35(8):441-448. [21] Lacerda SH, Semberova J, Holada K, et al. Carbon nanotubes activate store-operated calcium entry in human blood platelets. ACS Nano. 2011;5(7):5808-5813. [22] Patlolla A, Knighten B, Tchounwou P. Multi-walled carbon nanotubes induce cytotoxicity, genotoxicity and apoptosis in normal human dermal fibroblast cells. Ethn Dis. 2010;20(1):S1-65-72. [23] Patlolla A, Patlolla B, Tchounwou P. Evaluation of cell viability, DNA damage, and cell death in normal human dermal fibroblast cells induced by functionalized multiwalled carbon nanotube. Mol Cell Biochem. 2010;338(1-2): 225-232. [24] Cavallo D, Fanizza C, Ursini CL, et al.Multi-walled carbon nanotubes induce cytotoxicity and genotoxicity in human lung epithelial cells. J Appl Toxicol. 2012;32(6):454-464. [25] Hetrick EM, Schoenfisch MH. Reducing implant-related infections: active release strategies. Chem Soc Rev. 2006;35(9):780-789. [26] Sutherland IW. The biofilm matrix-an immobilised but dynamic environment. Trends Microbiol. 2001;9:222-227. [27] Nichols WW. Biofilms, antibiotics and penetration. Rev Med Microbiol. 1991;2:177-181. [28] Anderi JN, Franklin MJ, Stewart PS. Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicilin and ciprofloxacin, Antimicrob. Agents Chemother. 2000;44:1818-1824. [29] Bagge N, Ciofu O, Skocgaard LT, et al. Rapid development in vitro and in vivo of resistance to ceftazidimein biofilm-growing Pseudomonas aeruginosa due to chromosomal B-lactamase. APMIS 2000;108: 589-600. [30] Stoodley P, Ehrlich GD, Sedghizadeh PP, et al. Orthopaedic biofilm infections. Curr Orthop Pract. 2011;22(6):558-563. [31] Azam A, Ahmed AS, Oves M, et al.Size-dependent antimicrobial properties of CuO nanoparticles against Gram-positive and -negative bacterial strains. Int J Nanomedicine. 2012;7:3527-3535. [32] Tsao N, Luh TY, Chou CK, et al. In vitro action of carboxyfullerene. J Antimicrob Chemother. 2002;49(4):641-649. [33] Ercan B, Taylor E, Alpaslan E, et al. Diameter of titanium nanotubes influences anti-bacterial efficacy Nanotechnology. 2011;22(29):295102. [34] Puckett SD, Taylor E, Raimondo T, et al. Therelationship between the nanostructure of titanium surfaces and bacterial attachment. Biomaterials. 2010;31:706-713. [35] Popat KC, Eltgroth M, Latempa TJ, et al. Decreased Staphylococcus epidermis adhesion and increased osteoblast functionality on antibiotic-loadedtitania nanotubes. Biomaterials. 2007;28(32):4880-4888. [36] Zhang H, Sun Y, Tian A, et al. Improved antibacterial activity and biocompatibility on Gentamicin-loaded TiO2 nanotubes: in vivo and in vitro studies. Int J Nanomedicine. 2013;8:4379-4389. [37] Jia G, Wang H, Yan L, et al. Cytotoxicity of carbon nanomaterials: Single-wall nanotube, multiwall nantube, and fullerene. Environ Sci Technol. 2005;39:1378-1383. [38] Sayes CM, Liang F, Hudson JL, et al. Functionalization density dependence of single-walled carbon nanotubes cytotoxicity in vitro. Toxicol Lett. 2006;161(2):135-142. [39] Bottini M, Bruckner S, Nika K, et al. Multi-walled carbon nanotubes induce T lymphocyte apoptosis. Toxicol Lett. 2006;160:121-126. [40] Feng B, Weng J, Yang BC, et al. Characterization of titanium surfaces with calcium and phosphate and osteoblast adhesion. Biomaterials. 2005;25:3421-3428. [41] Oh SH, Finõnes RR, Daraio C, et al. Growth of nano-scale hydroxyapatite using chemically treated titanium oxide nanotubes. Biomaterials. 2005;26(24):4938-4943. [42] Kim HM, Himeno T, Kawashita M, et al. Surface potential change in bioactive titanium metal during the process of apatite formation in simulated body fluid. J Biomed Mater Res. 2003;67A(4): 1305-1309. [43] Bueno Rde B, Adachi P, Castro-Raucci LM, et al. Oxidative nanopatterning of titanium surfaces promotes production and extracellular accumulation of osteopontin. Braz Dent J. 2011; 22(3):179-184. [44] Jimbo R, Sotres J, Johansson C, et al. The biological response to three different nanostructures applied on smooth implant surfaces. Clin Oral Implants Res. 2012;23(6):706-712. [45] Liu Q, Huang S, Matinlinna JP, et al. Insight into Biological Apatite: Physiochemical Properties and Preparation Approaches. Biomed Res Int. 2013;2013:929748. [46] Pommer A, Muhr G, David A. Hydroxyapatite-coated Schanz pins in external fixators used for distraction osteogenesis: a randomized, controlled trial. J Bone Joint Surg Am. 2002;84-A(7): 1162-1166. [47] Johnson I, Akari K, Liu H. Nanostructured hydroxyapatite/poly(lactic-co-glycolic acid) composite coatingfor controlling magnesium degradation in simulated body fluid. Nanotechnology. 2013;24(37):375103. [48] D'Elía NL, Gravina AN, Ruso JM, et al. Manipulating the bioactivity of hydroxyapatite nano-rods structured networks: effects on mineral coatingmorphology and growth kinetic. Biochim Biophys Acta. 2013;1830(11):5014-5026. [49] Antonov EN, Bagratashvili VN, Popov VK, et al. Biocompatibility of Laser-deposited Hydroxyapatite Coatings on Titanium and Polymer Implant Materials. J Biomed Opt. 1998;3(4):423-428. [50] Shinto Y, Uchida A, Korkusuz F, et al. Calcium hydroxyapatite ceramic used as a delivery system for antibiotics. J Bone Joint Surg Br. 1992;74(4):600-604. [51] Krisanapiboon A, Buranapanitkit B, Oungbho K. Biocompatability of hydroxyapatite composite as a local drug delivery system. J Orthop Surg (Hong Kong). 2006;14(3):315-318. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [3] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [4] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [5] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [6] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [7] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [8] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [9] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [10] | Li Hui, Chen Lianglong. Application and characteristics of bone graft materials in the treatment of spinal tuberculosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 626-630. |
| [11] | Gao Cangjian, Yang Zhen, Liu Shuyun, Li Hao, Fu Liwei, Zhao Tianyuan, Chen Wei, Liao Zhiyao, Li Pinxue, Sui Xiang, Guo Quanyi. Electrospinning for rotator cuff repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 637-642. |
| [12] | Guan Jian, Jia Yanfei, Zhang Baoxin , Zhao Guozhong. Application of 4D bioprinting in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 446-455. |
| [13] | Huang Bo, Chen Mingxue, Peng Liqing, Luo Xujiang, Li Huo, Wang Hao, Tian Qinyu, Lu Xiaobo, Liu Shuyun, Guo Quanyi . Fabrication and biocompatibility of injectable gelatin-methacryloyl/cartilage-derived matrix particles composite hydrogel scaffold [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2600-2606. |
| [14] | Liu Jiali, Suo Hairui, Yang Han, Wang Ling, Xu Mingen. Influence of lay-down angles on mechanical properties of three-dimensional printed polycaprolactone scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2612-2617. |
| [15] | Li Xuan, Sun Yimin, Li Longbiao, Wang Zhenming, Yang Jing, Wang Chenglin, Ye Ling. Manufacturing of nano-modified polycaprolactone microspheres and its biological effects in dental pulp cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(10): 1530-1536. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||