Chinese Journal of Tissue Engineering Research ›› 2013, Vol. 17 ›› Issue (25): 4684-4691.doi: 10.3969/j.issn.2095-4344.2013.25.017
Previous Articles Next Articles
Preparation and characterization of a new kind of firm/soft adjusted poly(D,L-lactic acid)-polyurethane
Xu Jian2, Peng Kun1, 2, Gan Xiao-ling2 , Wang Yi-zheng2, Duan Qiao-ling2, Ruan Chang-shun1, Sun Jiao-xia1
- 1 Research Center of Bioinspired Material Science and Engineering, Department of Bioengineering, Chongqing University, Chongqing 400030, China
2 Department of Medical Technology, Chongqing Medical and Pharmaceutical College, Chongqing 401331, China
-
Received:2013-03-07Revised:2013-04-15Online:2013-06-18Published:2013-06-18 -
Contact:Peng Kun, Studying for doctorate, Lecturer, Research Center of Bioinspired Material Science and Engineering, Department of Bioengineering, Chongqing University, Chongqing 400030, China; Department of Medical Technology, Chongqing Medical and Pharmaceutical College, Chongqing 401331, China pk3001@163.com -
About author:Xu Jian, Associate professor, Department of Medical Technology, Chongqing Medical and Pharmaceutical College, Chongqing 401331, China -
Supported by:the Educational Department of Chongqing City, No. KJ112501; the Health Bureau of Chongqing City, No. 2012-2-257
CLC Number:
Cite this article
Xu Jian, Peng Kun, Gan Xiao-ling,Wang Yi-zheng, Duan Qiao-ling, Ruan Chang-shun, Sun Jiao-xia. Preparation and characterization of a new kind of firm/soft adjusted poly(D,L-lactic acid)-polyurethane [J]. Chinese Journal of Tissue Engineering Research, 2013, 17(25): 4684-4691.
share this article
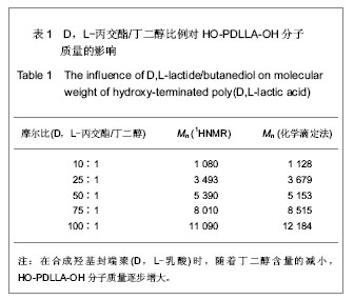
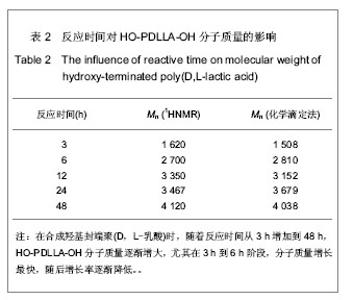
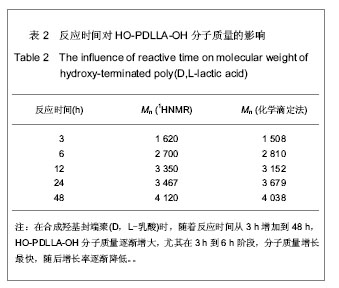
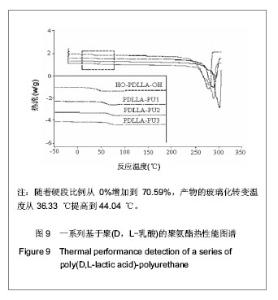
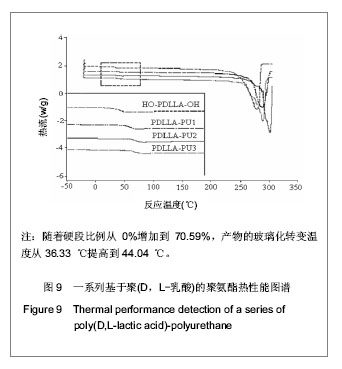
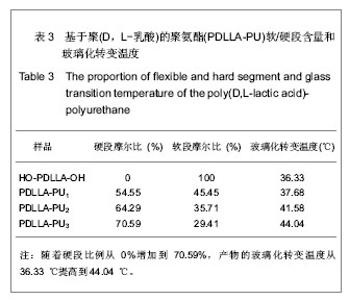
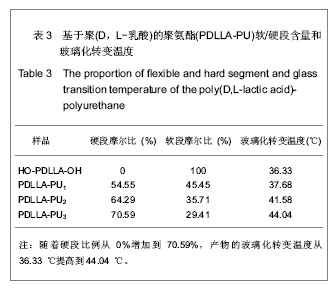
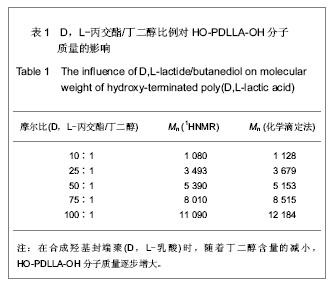
2.1 HO-PDLLA-OH的实验结果与讨论 2.1.1 反应物摩尔比及反应时间对HO-PDLLA-OH分子质量的影响 在反应时间为24 h的条件下,改变D,L-丙交酯/丁二醇的摩尔比,可合成不同分子质量的HO-PDLLA-OH。从反应产物的状态上看,当原料中丁二醇含量(丁二醇/D,L-丙交酯的摩尔比)由1∶100增加到1∶10时,产物的物理形态从坚硬的固体(一般分子质量较大)过渡到可拉伸的橡胶状体(一般分子质量较小)[23-24]。通过状态初步判断,改变丁二醇含量,达到了调节产物分子质量的目的。为进一步确定分子质量,在产物经过纯化、干燥后,利用1HNMR图谱和化学滴定法确定产物分子质量。实验结果见表1,随着丁二醇含量的减小,产物分子质量逐步增大;产物状态也随着丁二醇比例的增高而逐渐变硬、变脆。证实通过对引发剂丁二醇含量的调节,可以实现产物分子质量的调控。"
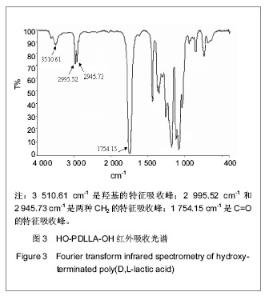
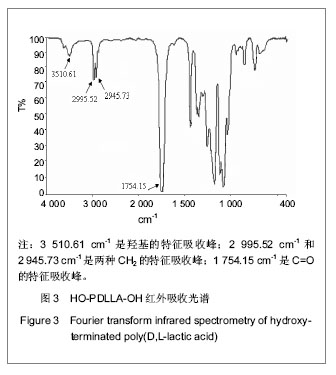
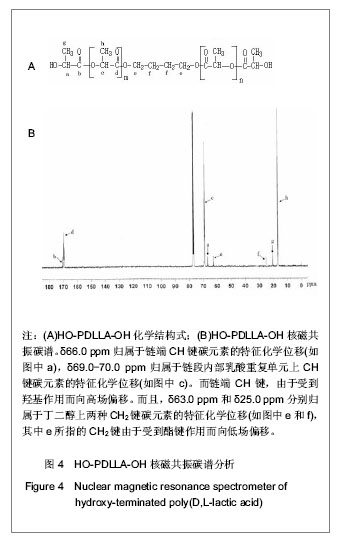
核磁共振碳谱分析:在反应时间为24 h,D,L-丙交酯/丁二醇(摩尔比)=25∶1的条件下,核磁共振碳谱分析见图4。可知:δ66.0 ppm归属于链端CH键碳元素的特征化学位移(如图4中a),δ69.0-70.0 ppm归属于链段内部乳酸重复单元上CH键碳元素的特征化学位移(如图4中c)。而链端CH键,由于受到羟基作用而向高场偏移。而且,δ63.0 ppm和δ25.0 ppm分别归属于丁二醇上两种CH2键碳元素的特征化学位移(如图4中e和f),其中e所指的CH2键由于受到酯键作用而向低场偏移。并且,图中没有发现羧基中碳元素的特征化学位移(理论上是δ165.8 ppm),证明该产物分子链上没有羧酸基团。上述结果进一步说明丙交酯与丁二醇发生聚合反应,而不是共混于产物中,并且产物分子链两端都应是羟基。"
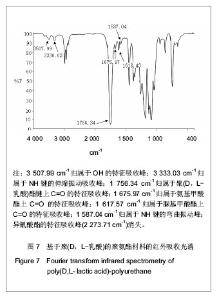
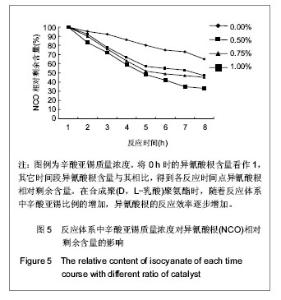
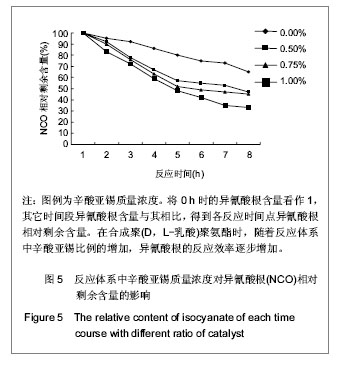
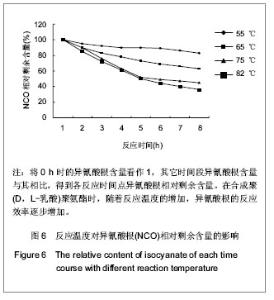
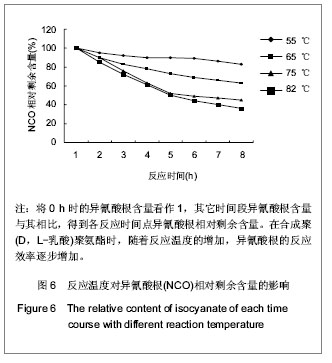
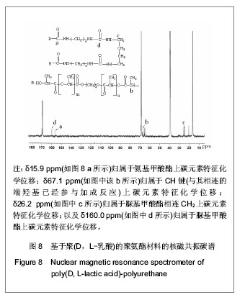
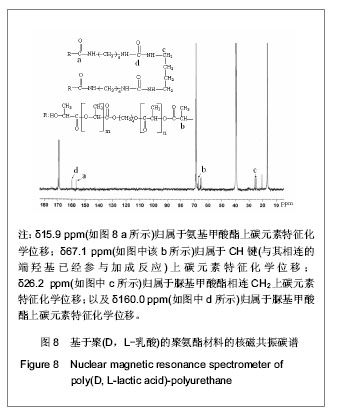
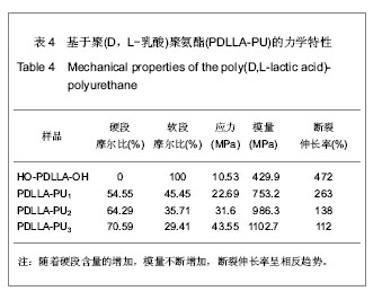
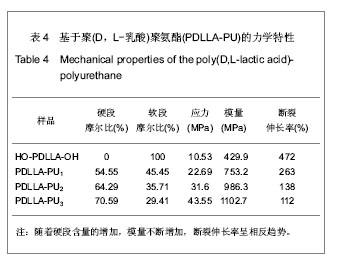
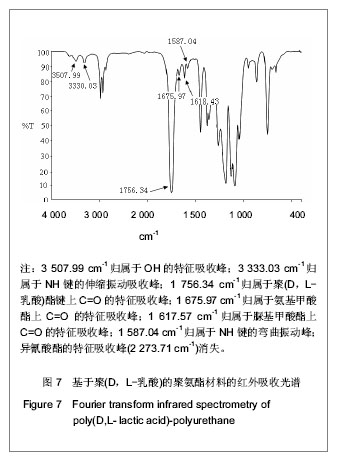
2.2.3 基于聚(D,L-乳酸)聚氨酯结构表征 红外吸收光谱分析:反应温度为75 ℃,催化剂辛酸亚锡比例为0.75%的条件下,红外吸收光谱分析见图7。可观察到如下特征峰:3 507.99 cm-1归属于OH的特征吸收峰;3 333.03 cm-1归属于NH键的伸缩振动吸收峰; 1 756.34 cm-1归属于聚(D,L-乳酸)酯键上C=O的特征吸收峰;1 675.97 cm-1归属于氨基甲酸酯上C=O的特征吸收峰;1 617.57 cm-1归属于脲基甲酸酯上C=O的特征吸收峰;1 587.04 cm-1归属于NH键的弯曲振动峰;异氰酸酯的特征吸收峰(2 273.71 cm-1)消失,意味着异氰酸酯被反应完全。"
| [1] Merline JD, Nair CPR, Gouri C, et al. Polyether polyurethanes: synthesis, characterization, and thermoresponsive shape memory properties. J Appl Polym Sci. 2008;107(6): 4082-4092.[2] Wang N, Dong A, Radosz M, et al. Thermoresponsive degradable poly(ethylene glycol) analogues. J Biomed Mater Res Part A. 2008;84(1):148-157.[3] Huang X, Nayak BR, Lowei TL. Synthesis and characterization of novel thermoresponsive-co-biodegradable hydrogels composed of N-isopropylacrylamide, poly(L-lactic acid), and dextran. J Polym Sci Part A: Polym Chem. 2004; 42(20):5054-5066.[4] Zhan JJ, Chen CH, Pan Y. Gaofenzi Tongbao. 2006;(8):21-28.湛建阶,陈朝辉,盘毅.形状记忆聚氨酯研究进展[J].高分子通报, 2006,(8):21-28.[5] Chen G, Imanishi YK, Ito Y. Effect of protein and cell behavior on pattern-grafted thermoresponsive polymer. J Biomed Mater Res. 1998;42(1):38-44.[6] Kaursoin J, Agrawal AK. Melt Spun Thermoresponsive shape memory fibers based on polyurethanes: effect of drawing and heat-setting on fiber morphology and properties. J Appl Polym Sci. 2007;103(4):2172-2182.[7] Watanabe E, Tomoshige N, Uyama H. New biodegradable and thermoresponsive polymers based on amphiphilic poly(asparagine) derivatives. Macromol Symp. 2007; 249-250(1):509-514.[8] Bian J, Lin HL , He FX , et al. Fabrication of microwave exfoliated graphite oxide reinforced thermoplastic polyurethane nanocomposites: Effects of filler on morphology, mechanical, thermal and conductive properties. Compos Part A-Appl S. 2013;47:72-82.[9] Sonnenschein MF , Ginzburg VV , Schiller KS, et al. Design, polymerization, and properties of high performance thermoplastic polyurethane elastomers from seed-oil derived soft segments. Polymer. 2013;54(4):1350-1360.[10] Liu C, Qin H, Mather PT. Review of progress in shape- memory polymers. J Mater Chem. 2007;17(16): 1543-1558. [11] Baer G., Wilson TS, Matthews DL, et al. Shape-memory behavior of thermally stimulated polyurethane for medical applications. J Appl Polym Sci. 2007;103(6):3882-3892.[12] Ni QQ, Zhang CS, Fu YQ, et al. Shape memory effect and mechanical properties of carbon nanotube/shape memory polymer nanocomposites. Compos Struct. 2007;81(2): 176-184.[13] da Silva RM, Mano JF, Reis RL. Smart thermoresponsive coatings and surfaces for tissue engineering: switching cell-material boundaries. Trends Biotechnol. 2007;25(12): 577-583.[14] Jiang XW, Xiong DA, An YL, et al. Thermoresponsive hydrogel of poly(glycidyl methacrylate-co-N- isopropylacrylamide) as a nanoreactor of gold nanoparticles. J Polym Sci Part A: Polym Chem. 2007;45(13):2812-2819.[15] Ajili SH, Ebrahimi NG, Soleimani M. Polyurethane/ polycaprolactane blend with shape memory effect as a proposed material for cardiovascular implants. Acta Biomaterialia. 2009;5(5):1519-1530.[16] Mehdi B, Khalid MZ, Ijaz AB, et al. Molecular engineering and properties of chitin based shape memory polyurethanes. Carbohydrate Polymers. 2008;74(3):621-626.[17] Gao LJ, Zhou LM , Fang SM , et al. New copoly(urethane-methacrylate)s obtained by adjusting the content of the poly(1,2-propanediol ortho-phthalate): Transparent, thermal, and mechanical properties. J Appl Polym Sci. 2013;128(6):3900-3905.[18] Saha S, Saha U, Singh JP, et al. Thermal and mechanical properties of homogeneous ternary nanocomposites of regioregular poly(3-hexylthiophene)-wrapped multiwalled carbon nanotube dispersed in thermoplastic polyurethane: Dynamic- and thermomechanical analysis. J Appl Polym Sci. 2013;128(3):2109-2120.[19] Heuchel M, Sauter T, Kratz K, et al. Thermally induced shape-memory effects in polymers: Quantification and related modeling approaches. J Polym Sci Pol Phys. 2013;51(8): 621-637.[20] Lendlein A, Zotzmann J, Feng Y, et al. Controlling the switching temperature of biodegradable, amorphous, shape-memory poly(rac-lactide)urethane networks by incorporation of different comonomers. Biomacromolecules. 2009;10(4):975-982.[21] Nagahama K, Ueda Y, Ouchi T, et al. Biodegradable shape-memory polymers exhibiting sharp thermal transitions and controlled drug release. Biomacromolecules. 2009;10(7): 1789-1794.[22] Behl M, Ridder U, Feng YK, et al. Shape-memory capability of binary multiblock copolymer blends with hard and switching domains provided by different components. Soft Matter. 2009; 5(3):676-684.[23] Zhang W, Chen L, Zhang Y. Surprising shape-memory effect of polylactide resulted from toughening by polyamide elastomer. Polymer. 2009;50(5):1311-1315.[24] Wong YS, Xiong Y, Venkatraman SS, et al. Shape memory in un-cross-linked biodegradable. J Biomater Sci Polym Ed. 2008; 19(2):175-191.[25] Wang YL, Ruan CS, Sun JX, et al. Degradation studies on segmented polyurethanes prepared with poly(D,L-lactic acid) diol, hexamethylene diisocyanate and different chain extenders. Polym Degrad Stabil. 2011;96(9):1687-1694.[26] Ruan CS, Wang YL, Zhang, ML , et al. Design, synthesis and characterization of novel biodegradable shape memory polymers based on poly(D,L-lactic acid) diol, hexamethylene diisocyanate and piperazine. Polym Inter. 2012;61(4): 524-530.[27] Kim HI, Ishihara K, Lee S, et al. Tissue response to poly(D,L-lactic acid)-based blend with phospholipid polymer for biodegradable cardiovascular stents. Biomaterials. 2011; 32(9):2241-2247. [28] Feng YK, Behl M, Kelch S, et al. Biodegradable multiblock copolymers based on oligodepsipeptides with shape-memory properties. Macromol Biosci. 2009;9(1):45-54. |
| [1] | Li Li, Ma Li. Immobilization of lactase on magnetic chitosan microspheres and its effect on enzymatic properties [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 576-581. |
| [2] | Zhou Anqi, Tang Yufei, Wu Bingfeng, Xiang Lin. Designing of periosteum tissue engineering: combination of generality and individuality [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3551-3557. |
| [3] | Lang Limin, He Sheng, Jiang Zengyu, Hu Yiyi, Zhang Zhixing, Liang Minqian. Application progress of conductive composite materials in the field of tissue engineering treatment of myocardial infarction [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3584-3590. |
| [4] | Cui Tiantian, Yi Lan, Ouyang Hougan, Wu Huiting, Ouyang Yanchu, Chen Chu. Effect of thermosensitive moxibustion in a rat model of pelvic inflammation based on trifocal focal membrane theory [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(20): 3168-3172. |
| [5] | Xie Jian, Su Jiansheng. Advantages and characteristics of electrospun aligned nanofibers as scaffolds for tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(16): 2575-2581. |
| [6] | Ji Qi, Yu Zhengwen, Zhang Jian. Problems and trends of technique and clinical application of metallic biomaterials prepared by three-dimensional printing technology [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(16): 2597-2604. |
| [7] | Qian Nannan, Zhang Qian, Yang Rui, Ao Jun, Zhang Tao. Mesenchymal stem cells in the treatment of spinal cord injury: cell therapy and combination of new drugs and biomaterials [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(13): 2114-2120. |
| [8] | Jia Wei, Zhang Mandong, Chen Weiyi, Wang Chenyan, Guo Yuan. Effects of femoral prosthetic materials on artificial knee arthroplasty performance [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(10): 1477-1481. |
| [9] | Wang Qian, Li Lu, Shu Jingyuan, Dong Zhiheng, Jin Youshi, Wang Qingshan. Micro-morphology and phase of zirconia-based nano-hydroxyapatite functional gradient biomaterials [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(10): 1517-1521. |
| [10] | Li Li, Ma Li, Li He. Preparation and characterization of magnetic chitosan microspheres [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(4): 577-582. |
| [11] | Tang Mengmeng, Chen Hechun, Xie Hongchen, Zhang Yu, Tan Xiaoshuang, Sun Yixuan, Huang Yina. Histocompatibility of poly(L-lactide-co-ε-caprolactone)/cross-linked polyvinylpyrrolidone ureteral stent grafted into the rat bladder [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(4): 583-588. |
| [12] | Chen Yongjia, Li Yanlin, Liu Dejian, He Yinghong, Yang Xiao. Sustained-release effect and clinical application of thermosensitive gels [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(34): 5428-5433. |
| [13] | Guo Enhui, Xu Zitong, Liang Yize, Zhou Liang, Lu Zhaoxiang, You Liang, Xia Yujun. Properties of a novel photocrosslinked fish collagen peptide-hyaluronic acid hydrogel [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(28): 4518-4525. |
| [14] | Xin Pengfei, Sun Youqiang, Li Jie, Chen Jianfa, Deng Baogui, Xiang Xiaobing . Research progress of biomaterials for repair of rotator cuff tear [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(28): 4459-4464. |
| [15] | Liu Yanhua, Zhu Zhou, Wan Qianbing. A drug-loading system for electrospinning wound repair: component selection and construction strategy [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(28): 4465-4473. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||