Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (31): 5042-5047.doi: 10.12307/2024.701
Previous Articles Next Articles
miRNA derived from mesenchymal stem cells and its derivatives in treatment of pathological scar
Li Yue1, 2, Qiao Hua1
- 1School of Basic Medicine, Shanxi Medical University, Taiyuan 030001, Shanxi Province, China; 2First Clinical Medical College of Shanxi Medical University, Taiyuan 030001, Shanxi Province, China
-
Received:2023-09-07Accepted:2023-10-13Online:2024-11-08Published:2024-01-22 -
Contact:Qiao Hua, MD, Associate professor, School of Basic Medicine, Shanxi Medical University, Taiyuan 030001, Shanxi Province, China -
About author:Li Yue, School of Basic Medicine, Shanxi Medical University, Taiyuan 030001, Shanxi Province, China; First Clinical Medical College of Shanxi Medical University, Taiyuan 030001, Shanxi Province, China -
Supported by:Innovation and Entrepreneurship Training Program for College Students in Shanxi Province, No. 20230295 (to LY); Youth Science and Technology Research Fund of Shanxi Provincial Department of Science and Technology, No. 201901D211339 (to QH)
CLC Number:
Cite this article
Li Yue, Qiao Hua. miRNA derived from mesenchymal stem cells and its derivatives in treatment of pathological scar[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(31): 5042-5047.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
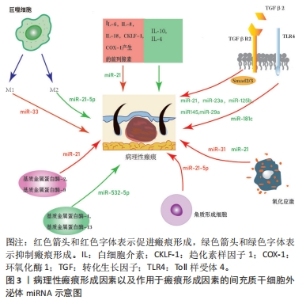
2.1 MSC-Exos及其miRNA成分的生物学特性 2.1.1 MSCs MSCs是一类具有自我更新和多谱系分化能力的多能成体干细胞,具有抗炎、促进血管生成以及调节细胞外基质重塑等作用[4],其来源广泛,易于获取,体外可大量扩增,且免疫原性低下,是目前组织修复中首选的种子细胞[5]。 2.1.2 MSC-Exos MSCs旁分泌过程中能产生Exos。Exos是一种细胞外囊泡,由多囊泡体与细胞膜融合后向胞外分泌[6]。Exos直径为30-200 nm,存在于各种体液中,几乎所有细胞都能分泌Exos,其内富含mRNA、miRNA及蛋白质等活性成分,能与胞膜表面受体通过结合、胞吞或膜融合等多种机制被靶细胞摄取[7-8]。Exos在细胞间通信中起重要作用,能在细胞之间运输miRNA并随后调节靶细胞中基因的表达[9]。YU等[10]指出MSC-Exos具有来源细胞相似的一些功能,如调控创伤局部炎症反应、免疫反应、血管新生和抗凋亡等,通过间接作用修复病变缺损的组织器官。MSC-Exos和MSCs的miRNA表达谱相似但表达量不同,且MSC-Exos比MSCs有更好的治疗效果,在某些方面可以取代干细胞治疗[11]。 2.1.3 MSC-Exos中的miRNA miRNA是20-23个核苷酸(nt)长度的小分子非编码RNA[12]。通过与其靶基因mRNA 3’非编码区互补配对,以RNA干扰的方式调节转录后基因的表达而调节相应细胞增殖、凋亡和迁移[13-14]。除了内源性表达外,miRNA还可以分泌到细胞外,与称为Exos的脂质载体有关。循环中含有miRNA的Exos已被证明在体外被受体细胞吸收并通过RNA干扰改变基因表达[13]。 为响应不同的功能要求和外部干预状态,MSC-Exos中的miRNA根据宿主细胞的周围微环境和代谢状态不断变化[15]。上述分析表明,MSC-Exos通过向靶细胞运输特定miRNA重编程靶细胞基因的表达,调节靶细胞的增殖、凋亡和迁移,从而调节皮肤组织修复过程。 2.2 MSC-Exos衍生的miRNA治疗病理性瘢痕相关的机制 2.2.1 MSC-Exos衍生的miRNA通过抗炎进而抑制瘢痕病变 (1)病理性瘢痕病变过程中的炎症反应过程:炎症是机体对有害刺激作出反应的一种自我防御机制,它的强度和持续时间影响瘢痕的大小。肌成纤维细胞收缩能力强,能分泌大量与瘢痕相关的细胞外基质。在组织重塑阶段,若出现过度炎症反应,炎症细胞和促炎因子积累过多,则成纤维细胞向肌成纤维细胞的转化会失去控制。通常肌成纤维细胞在伤口闭合后通过细胞凋亡从肉芽组织中消失,而成纤维细胞向肌成纤维细胞的转化若持续存在则会导致创面的过度收缩引发病理性瘢痕[16]。 巨噬细胞的极化:伤口愈合过程中,巨噬细胞的不平衡极化是导致病理性瘢痕形成的主要原因[17]。巨噬细胞是重要的炎症细胞,随微环境不同,其可以极化成2种表型:由Toll样受体与T辅助细胞1(Th1)信号诱导的经典活化巨噬细胞(M1),以及由T辅助细胞2(Th2)信号激活的替代活化巨噬细胞(M2)[18-19],见图3。在伤口愈合过程中,促炎M1型巨噬细胞启动炎症反应,而抗炎M2型巨噬细胞参与增殖和重塑阶段,释放多种生长因子,如血管内皮生长因子、血小板衍生生长因子和转化生长因子β等,这些生长因子加快向增殖期的转变[20],见图3。损伤后早期M1细胞因子表达减少,或后期M2和抗炎细胞因子表达延迟和延长,都可能导致病理性瘢痕的形成[17]。"
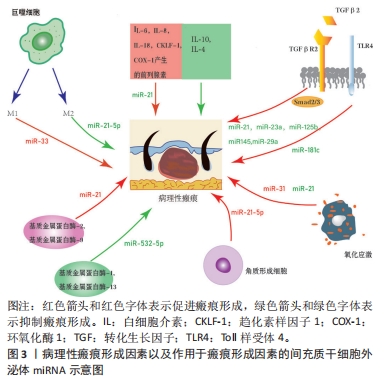
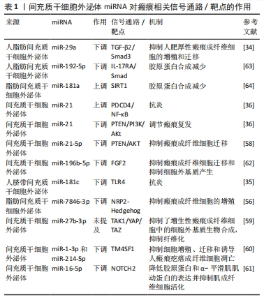
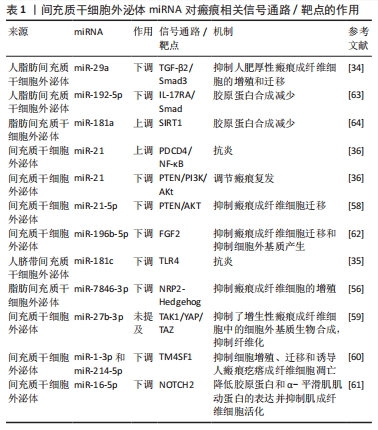
转化生长因子β失调:转化生长因子β是一种炎症因子,在炎症反应中起着至关重要的作用。通常M2型巨噬细胞通过分泌转化生长因子β促进成纤维细胞向肌成纤维细胞的转化,促进伤口纤维化[21]。病理情况下,转化生长因子β1等炎症递质过度表达,会过度激活瘢痕疙瘩成纤维细胞,进而增加细胞外基质胶原合成,导致瘢痕形成[22]。 此外,Toll样受体可以通过如图3所示的TLRs-TGF-β-Smads信号通路,参与瘢痕组织纤维化。瘢痕疙瘩中Toll样受体4的高表达通过Smad4信号通路增加转化生长因子β、结缔组织生长因子和胶原的表达;Smad7的激活可抑制瘢痕形成,因此上调Smad7可能减少转化生长因子β1表达和抑制纤维化[23]。 炎性细胞因子的不平衡:在伤口愈合的任何阶段细胞因子的不平衡都可能导致异常瘢痕形成。过度和长时间的促炎反应诱导病理性瘢痕的发生。在瘢痕疙瘩组织中发现大量因子,包括白细胞介素6、白细胞介素8、白细胞介素18、趋化素样因子1,由环加氧酶1产生的前列腺素等在组织损伤时表现出促炎作用,且含量显著增高;趋化因子CXCL12诱导小鼠瘢痕增大,白细胞介素17在增生性瘢痕组织中升高,巨噬细胞介导白细胞介素17促纤维化作用。此外,对伤口愈合产生抗炎作用的因子通常被认为有利于预防瘢痕形成。白细胞介素10已被证明对胶原蛋白合成产生负调节作用,最终减少瘢痕形成,白细胞介素10的抗炎和抗瘢痕作用也在基因敲除动物中得到验证,同时缺乏白细胞介素10和白细胞介素4的小鼠表现出促炎症反应和瘢痕恶化。相比之下,在伤口区域补充白细胞介素10可减轻瘢痕形成[2],见图3。 (2) MSC-Exos衍生的miRNA抑制炎症从而抑制瘢痕病变 调控巨噬细胞:脂多糖能诱导炎症微环境,SHEN等[24]将MSC-Exos与脂多糖诱导的RAW264.7细胞共孵育,发现MSC-Exos促进该细胞向M2表型极化并上调其标记物表达水平。经抑制miR-21-5p表达后,MSC-Exos促进了脂多糖诱导的RAW264.7细胞向M1表型极化,并上调了培养液上清中炎症因子水平;此外还发现,转染miR-21-5p模拟物促进了RAW264.7细胞向M2表型极化,并降低了培养上清液中炎症因子水平,这说明MSC-Exos中的miR-21-5p可以促进巨噬细胞向M2表型极化,从而减少炎症因子表达,见图3。同时,其他学者发现,脂多糖预处理MSC-Exos促进了M2巨噬细胞的激活,利于缓解炎症,加快伤口愈合[25];Exos可以将miR-33递送到巨噬细胞中,诱导M1极化[26],引起炎症反应,见图3。 调控转化生长因子β:大量研究表明,TGF-β/SMADs信号通路是某些特定miRNA(如miR-21、miR-27、miR-92、miR-217、miR-587)的靶基因,并受其调控[27-31]。JIN等[32]发现长链非编码RNA HOXA11-AS通过miR-124-3p/TGFβR1轴抑制细胞凋亡并促进成纤维细胞诱导的血管生成,miR-124-3p直接靶向TGFβR1参与HOXA11-AS介导的表型,从而调节PI3K/Akt信号通路,这将为瘢痕疙瘩治疗提供新的靶点。 此外,研究显示miRNA修饰的MSC-Exos可以通过干扰炎症反应进而抑制瘢痕[13]。其中人脐带MSC-Exos中的特异miRNA如miR-21、miR-23a、miR-125b和miR-145可以通过抑制转化生长因子β2、转化生长因子β受体2以及Smad2引起α-平滑肌肌动蛋白的下调,从而抑制成纤维细胞向肌成纤维细胞的转化[33],见图3。YUAN等[34]发现用miR-29a修饰的人脂肪MSC-Exos处理后抑制了人肥厚性瘢痕成纤维细胞的增殖和迁移,同时发现转化生长因子β2是miR-29a的靶标,总结得出人脂肪MSC-Exos衍生的外源性miR-29a通过抑制TGF-β2/Smad3信号通路来抑制人肥厚性瘢痕成纤维细胞的纤维化和瘢痕增生,见图3。LI等[35]发现人脐带MSC-Exos中的miR-181c通过下调TLR4信号通路来减少烧伤诱导的炎症,说明MSCs衍生的外源性miRNA调节TLRs-TGF-β-Smads信号通路可能是减少炎症、抑制瘢痕增生的一种有前途的方法,见图3。 调控炎性细胞因子:PDCD4是受miR-21调节的肿瘤抑制因子,通过阻断蛋白质翻译对细胞增殖具有抑制作用。NF-κB通过促进肿瘤坏死因子α和白细胞介素6的表达并抑制白细胞介素10的表达来促进炎症。抑制miR-21表达增加了PDCD4表达,诱导了NF-κB的活化,最终导致促炎细胞因子的合成增加。因此上调miR-21/PDCD4/NF-κB信号通路可以发挥抗炎作用。miR-21也可以通过抑制激活PI3K/Akt信号通路的PTEN促进伤口愈合或调节瘢痕疙瘩复发[36],见图3。 因此,MSC-Exos高表达的miRNAs通过促进巨噬细胞极化增加M2型巨噬细胞比例,靶向调节转化生长因子β、转化生长因子β受体或TGF-β/Smad信号通路,抑制促炎因子的产生、促进抗炎因子的表达等机制,在抑制炎症反应进而抑制瘢痕病变方面发挥积极作用。 2.2.2 MSC-Exos衍生的miRNA通过抑制过度组织重塑进而抑制瘢痕病变 (1)病理性瘢痕病变过程中的组织重塑过程:胶原平衡的异常调节导致病理性瘢痕形成异于正常瘢痕的胶原束和纤维,这些胶原束和纤维由许多紧密堆积在一起的胶原纤维组成,比正常皮肤、正常营养性瘢痕中的纤维更厚[37-38]。胶原平衡的异常调节导致胶原在体内瘢痕病变中沉积有2种可能机制:一是基质金属蛋白酶失调导致胶原多肽的降解减少,二是成纤维细胞对细胞外基质的调控失常导致蛋白质产物之间不平衡。因此调控基质金属蛋白酶促进胶原多肽的降解与调控成纤维细胞对细胞外基质的作用是抑制过度组织重塑的2种机制。 (2)基质金属蛋白酶失调导致瘢痕病变 基质金属蛋白酶与其抑制剂的表达失衡减少胶原多肽降解:细胞外基质由胶原、弹力素及明胶等组成,可被众多蛋白裂解酶所降解,基质金属蛋白酶就是其中之一。基质金属蛋白酶是一类含锌内肽酶,是基质降解的主要酶系,在伤口愈合过程中的胶原降解、细胞外基质重塑以及瘢痕组织形成中发挥关键作用[39]。许多参与瘢痕形成的基质金属蛋白酶是由成纤维细胞本身分泌的[40]。基质金属蛋白酶1是一种间质胶原酶,能破坏胶原蛋白的三螺旋结构,分解细胞基质粘连,从而促进上皮再皮化过程[41];基质金属蛋白酶2和基质金属蛋白酶9是明胶酶蛋白,可降解明胶并去除已被胶原酶切割的异常或未折叠的胶原蛋白[42]; 基质金属蛋白酶3是一种基质溶酶蛋白,可以切割胶原蛋白和非胶原蛋白分子,如蛋白聚糖、层粘连蛋白和纤连蛋白,从而影响伤口收缩[43]。基质金属蛋白酶抑制剂是基质金属蛋白酶的内源性天然抑制剂,可以通过与基质金属蛋白酶形成1∶1络合物抑制基质金属蛋白酶活性,阻止细胞外基质的降解。因此,基质金属蛋白酶及基质金属蛋白酶抑制剂的表达一旦失去平衡,基质的合成代谢和分解代谢就会失去平衡,就可能触发病理性瘢痕的形成。 基质金属蛋白酶促进成纤维细胞迁移:增生性瘢痕来源成纤维细胞依赖基质金属蛋白酶进行转移。增生性瘢痕形成早期,创面愈合还未完成,基质金属蛋白酶分泌增加,促进成纤维细胞、肌成纤维细胞的迁移而引发异常细胞外基质降解;随着瘢痕的发展,基质金属蛋白酶抑制剂分泌相对增加,基质金属蛋白酶抑制剂与基质金属蛋白酶表达失去平衡,抑制成纤维细胞的迁移、增殖,进而抑制基质的降解,细胞外基质在细胞外累积,形成增生性瘢痕[44]。此外,有研究表明瘢痕疙瘩成纤维细胞也依赖于基质金属蛋白酶进行迁移。瘢痕疙瘩成纤维细胞中基质金属蛋白酶表达量升高,促进了瘢痕疙瘩成纤维细胞的迁移,进而促进瘢痕疙瘩的形成,说明基质金属蛋白酶对瘢痕疙瘩成纤维细胞的迁移活动具有重要作用[45]。XUE等[19]发现瘢痕疙瘩成纤维细胞培养过程中胶原蛋白和基质金属蛋白酶的分泌量增加,且转化生长因子β1通过诱导平滑肌肌动蛋白、基质金属蛋白酶2、基质金属蛋白酶9的过表达激活肌成纤维细胞,同时抑制基质金属蛋白酶1表达,从而导致胶原过度积累,导致病理性瘢痕形成,见图3。 (3)成纤维细胞失调导致瘢痕病变:生理条件和纤维化的大多数研究表明,表皮角质形成细胞可以与下层真皮的成纤维细胞通过表皮-间充质通讯相互作用,调节组织稳态和修复[46]。研究表明,角质形成细胞可能参与增强细胞外基质沉积[47]。NOWINSKI等[48]通过建立角质形成细胞-成纤维细胞共培养系统并利用大规模微阵列分析,证明成纤维细胞中编码生长因子、细胞因子及其受体、细胞外基质、黏附受体、基质金属蛋白酶和细胞周期调节因子在体外受到角质形成细胞衍生因子的调节,同样程度的细胞间通讯也可能存在于体内,有助于损伤后组织完整性的重建。FUNAYAMA等[49]利用无血清间接共培养系统研究了正常皮肤和瘢痕疙瘩来源角质形成细胞对正常皮肤和瘢痕疙瘩来源成纤维细胞的影响,结果表明瘢痕疙瘩来源角质形成细胞通过旁分泌和双旁分泌效应,导致转化生长因子β1、MAPK磷酸化和Bcl-2表达增加,促进下层成纤维细胞增殖,细胞凋亡减少,由此细胞凋亡和增殖之间平衡破坏可能导致瘢痕疙瘩的形成。MA等[50]通过过氧化氢处理角质形成细胞建立皮肤损伤模型,并提出脂肪MSCs通过激活Wnt/β-catenin信号通路促进受损角质形成细胞的迁移,以调节细胞外基质的重塑。YAN等[51]发现miR-21-5p可以通过靶向PTEN基因调节AKT信号通路,以进一步影响瘢痕疙瘩角质形成细胞的上皮间充质转化表型和干性特征,导致瘢痕疙瘩侵袭和复发,表明miR-21-5p及其相关的上皮间充质转化表型和AKT信号通路可能是瘢痕疙瘩的新型治疗靶点,见图3。 尽管已经有大量研究证明在瘢痕发病机制中角质形成细胞促进成纤维细胞增殖并抑制其凋亡,以及MSCs、miRNA对角质形成细胞产生影响[52-53],但目前仍然缺乏MSC-Exos的miRNA影响角质形成细胞的相关研究。 (4) MSC-Exos中miRNA通过抑制过度组织重塑从而抑制瘢痕病变 调控基质金属蛋白酶:YANG等[54]研究发现,脂肪MSC-Exos中高度表达miR-21,通过PI3K/AKT信号通路调节基质金属蛋白酶2的表达,增强人永生化角质形成细胞(HaCaT)的迁移和增殖,说明MSC-Exos中miRNA可以靶向基质金属蛋白酶影响促进胶原合成的细胞增殖、迁移。ZHU等[55]研究表明骨髓MSC-Exos来源miR-532-5p通过靶向退化髓核细胞中的基质金属蛋白酶13来抑制基质降解,见图3。 调控成纤维细胞:脂肪MSC-Exos miR-7846-3p通过抑制NRP2-Hedgehog轴来抑制瘢痕疙瘩成纤维细胞的增殖和促进血管生成[56]。 MSCs-Exo中的miR-138-5p可以通过靶向沉默信息调节因子1(silent information regulator 1,SIRT1)来减轻病理性瘢痕,并进一步抑制人类瘢痕成纤维细胞的增殖、迁移和纤维化蛋白表达[57]。YAN等[58]研究发现miR-21-5p通过抑制电子束照射的瘢痕疙瘩成纤维细胞中的磷酸酯酶与张力蛋白同源物(phosphatase and tensin homolog,PTEN)/Akt信号传导调节迁移和自噬,防止局部侵袭和复发。LI等[59]发现miR-27b-3p的过表达显著抑制了增生性瘢痕成纤维细胞中的细胞外基质生物合成,通过miR-27b-3p/TAK1/YAP/TAZ轴显著抑制了增生性瘢痕中的纤维化。XU等[60]研究表明与增生性瘢痕成纤维细胞相比,四次跨膜蛋白1(transmembrane 4 L six family 1,TM4SF1)在人瘢痕疙瘩成纤维细胞中上调。TM4SF1的下调显著抑制了人瘢痕疙瘩成纤维细胞的增殖和迁移,并诱导了细胞凋亡。miR-1-3p和miR-214-5p靶向抑制TM4SF1表达,抑制人瘢痕疙瘩成纤维细胞的增殖和迁移并诱导其凋亡。YAO等[61]发现缺口受体 2(notch receptor 2 Gene,NOTCH2)活化促进了体外纤维化进展,而miR-16-5p能够直接抑制NOTCH2,降低胶原蛋白和α-平滑肌肌动蛋白的表达并抑制肌成纤维细胞活化。miR-196b-5p直接靶向成纤维细胞生长因子2,抑制瘢痕疙瘩成纤维细胞活力、迁移和细胞外基质蛋白产生[62] 。 直接减少胶原蛋白产生:miR-192-5p靶向抑制白细胞介素17受体A的表达促进了伤口愈合,减弱了胶原蛋白的产生,并调节了增生性瘢痕成纤维细胞中的Smad通路。通过miR-192-5p/IL-17RA/Smad轴可以减轻肥厚性瘢痕纤维化[63]。SIRT1被确认为miR-181a的下游靶标之一,注射SIRT1抑制剂导致胶原蛋白合成增加,脂肪MSC-Exos有望通过调节miR-181a/SIRT1轴来拮抗瘢痕形成[64]。 因此,MSCs-Exos高表达的miRNAs可以通过减少基质金属蛋白酶分泌,调节基质金属蛋白酶抑制剂与基质金属蛋白酶的平衡,抑制成纤维细胞、肌成纤维细胞的增殖迁移,直接减少胶原蛋白的产生等机制最终使得细胞外基质正常降解,在抑制过度的组织重塑而抑制瘢痕病变方面发挥积极作用。 2.2.3 MSC-Exos衍生的miRNA通过抗氧化进而抑制瘢痕病变 (1)氧化应激导致瘢痕病变:缺氧微环境为瘢痕疙瘩角质形成细胞通过上皮间充质转化呈现成纤维细胞样外观提供了有利的环境,增强了瘢痕疙瘩角质形成细胞的侵袭能力,并使瘢痕疙瘩延伸到伤口边缘之外[65]。缺氧微环境有利于活性氧的增加,活性氧过度积累则导致氧化应激[66]。大量研究发现,瘢痕疙瘩组织中的氧化应激显著增加,血浆中的抗氧化剂显著减少,提示氧化/抗氧化失衡可能在皮肤纤维化中发挥重要作用。氧化应激可导致瘢痕疙瘩成纤维细胞中活性氧、NADPH氧化酶和Ⅰ型胶原的表达增加,表明氧化应激介导了人瘢痕疙瘩成纤维细胞的迁移和细胞外基质的合成[67],见图3。此外,缺氧可诱导PI3K/Akt和ERK1/2信号通路激活,使缺氧诱导因子1α在瘢痕疙瘩中积累,激活纤维化信号通路,最终导致纤维化加重和浸润性生长[65]。当PI3K/AKT通路受到抑制时,活性氧水平显著升高,瘢痕疙瘩成纤维细胞的增殖和迁移受到抑制而凋亡增加。PI3K/AKT通路通过调节糖酵解促进缺氧下瘢痕疙瘩成纤维细胞的增殖并抑制细胞凋亡,表明PI3K/AKT信号通路可能是瘢痕疙瘩的治疗靶点[68]。转化生长因子β1可通过调控瘢痕疙瘩中的Smad通路和促进瘢痕疙瘩成纤维细胞的纤维化表型来促进EGR1的表达,EGR1可以通过靶向NOX4来调节活性氧的产生。此外,NOX4衍生的活性氧可促进瘢痕疙瘩成纤维细胞的纤维化样表型,在瘢痕疙瘩纤维化中发挥重要作用。TGF-β1/EGR1/NOX4轴可能作为瘢痕疙瘩的潜在治疗靶点[69]。 (2) MSC-Exos中miRNA通过抗氧化从而抑制瘢痕病变:WU等[70]研究发现瘢痕疙瘩组织中miR-21水平升高,miR-21的上调导致活性氧降低,而miR-21的抑制增加活性氧水平,表明miR-21可能诱导瘢痕疙瘩组织对氧化应激的抵抗力,miR-21可作为瘢痕疙瘩成纤维细胞的潜在治疗靶点,见图3。ZHANG等[71]发现miR-31的下调通过靶向缺氧诱导因子1的负调节剂HIF1AN来抑制细胞增殖,诱导细胞凋亡并干扰细胞周期进程,HIF1AN被确认为是miR-31的靶标。进一步的研究表明,miR-31通过介导HIF1AN/VEGF信号通路来调节瘢痕疙瘩来源成纤维细胞的增殖、凋亡和细胞周期,因此miR-31为瘢痕疙瘩的有希望的治疗靶点,见图3。 MSC-Exos高表达的miRNAs可以通过调节活性氧和缺氧诱导因子等途径提高瘢痕成纤维细胞对氧化应激的抵抗力,进而调节瘢痕成纤维细胞的增殖、凋亡从而抑制瘢痕病变。 MSC-Exos的miRNA对瘢痕相关信号通路/靶点的作用,见表1。"

值得注意的是,瘢痕发展中的新的治疗靶点和潜在的生物标志物可以通过高通量测序技术和生物信息学分析技术筛选。通过分析GSE113620数据集中瘢痕疙瘩中异常表达的miRNA,并查询生物信息学系统中含有脂肪MSC-Exos的miRNA,WU等[56]发现了miR-7846-3p作为外泌体miRNA在瘢痕疙瘩中异常表达,随后观察到其在脂肪MSC-Exos中的特异性抑制阻断了其抑制增殖和血管生成作用。通过查询4个生物信息学系统,获得了NRP2作为miR-7846-3p的靶标。而先前工作揭示了NRP2促进人脐静脉内皮细胞的迁移以诱导血管生成,NRP2-Hedgehog轴与巨噬细胞的M2极化和人真皮成纤维细胞的增殖有关,Hedgehog途径相关分子的激活与瘢痕疙瘩发育的细胞生长和增殖有关。通过实验发现脂肪MSC-Exos处理降低了NRP2的水平,NRP2在人瘢痕疙瘩成纤维细胞中的过表达否定了Exos的功能。因此,可以认为NRP2-Hedgehog信号通路的抑制与脂肪MSC-Exos的治疗作用有关。LV等[72]从基因表达综合(GEO)数据库下载了微阵列数据集GSE113620,以筛选出miRNA(DEMs)的差异表达;应用大方差的差异表达基因构建加权基因共表达网络,以鉴定与瘢痕疙瘩进展密切相关的miRNA模块;最后miR-424-3p被认为是与瘢痕疙瘩进展最相关的最终目标,通过分子生物学实验结果确定miR-424-3p靶向Smad7后细胞增殖、迁移和胶原蛋白分泌能力显著增强,而细胞凋亡率显著降低。HE等[73]从GEO和ArrayExpress数据库中筛选了6个数据集,包括20例瘢痕疙瘩患者,严格控制样本组和对照组,并在一定程度上扩大样本量,以GSE113619和GSE113620数据集分别作为验证数据集验证mRNA和miRNA的表达水平,采用RT-qPCR验证miRNA-mRNA网络,以提高结果的可靠性,降低假阳性率,筛选出了真正的中枢基因miR-29a-3p,初步验证了miR-29a-3p的潜在靶点,为瘢痕疙瘩的治疗提供了潜在的治疗。"

| [1] RIEDEMANN HI, SCHMIDT MF, BARON JM. Therapy of pathological scars. J Dtsch Dermatol Ges. 2023;21(7):761-776. [2] WANG ZC, ZHAO WY, CAO Y, et al. The Roles of Inflammation in Keloid and Hypertrophic Scars. Front Immunol. 2020;11:603187. [3] LINGZHI Z, MEIRONG L, XIAOBING F. Biological approaches for hypertrophic scars. Int Wound J. 2020;17(2):405-418. [4] LEE DE, AYOUB N, AGRAWAL DK. Mesenchymal stem cells and cutaneous wound healing: novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Res Ther. 2016;7:37. [5] ZHAO G, GE Y, ZHANG C, et al. Progress of Mesenchymal Stem Cell-Derived Exosomes in Tissue Repair. Curr Pharm Des. 2020;26(17):2022-2037. [6] MASHOURI L, YOUSEFI H, AREF AR, et al. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 2019;18(1):75. [7] PEGTEL DM, GOULD SJ. Exosomes. Annu Rev Biochem. 2019;88:487-514. [8] ZAREI F, ABBASZADEH A. Stem cell and skin rejuvenation. J Cosmet Laser Ther. 2018;20(3):193-197. [9] SHIMBO K, MIYAKI S, ISHITOBI H, et al. Exosome-formed synthetic microRNA-143 is transferred to osteosarcoma cells and inhibits their migration. Biochem Biophys Res Commun. 2014;445(2):381-387. [10] YU B, ZHANG X, LI X. Exosomes derived from mesenchymal stem cells. Int J Mol Sci. 2014;15(3):4142-4157. [11] SHAO L, ZHANG Y, LAN B, et al. MiRNA-Sequence Indicates That Mesenchymal Stem Cells and Exosomes Have Similar Mechanism to Enhance Cardiac Repair. Biomed Res Int. 2017;2017:4150705. [12] FARAZI TA, SPITZER JI, MOROZOV P, et al. miRNAs in human cancer. J Pathol. 2011; 223(2):102-115. [13] MOBERGSLIEN A, SIOUD M. Exosome-derived miRNAs and cellular miRNAs activate innate immunity. J Innate Immun. 2014;6(1):105-110. [14] MALET H, LORENTZEN E. Mechanisms of RNA recruitment by the exosome. RNA Biol. 2011;8(3):398-403. [15] DENG H, SUN C, SUN Y, et al. Lipid, Protein, and MicroRNA Composition Within Mesenchymal Stem Cell-Derived Exosomes. Cell Reprogram. 2018;20(3):178-186. [16] NANGOLE FW, AGAK GW. Keloid pathophysiology: fibroblast or inflammatory disorders? JPRAS Open. 2019;22:44-54. [17] XU X, GU S, HUANG X, et al. The role of macrophages in the formation of hypertrophic scars and keloids. Burns Trauma. 2020;8:tkaa006. [18] GORDON S, MARTINEZ FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593-604. [19] XUE M, JACKSON CJ. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv Wound Care (New Rochelle). 2015;4(3):119-136. [20] NOVAK ML, KOH TJ. Macrophage phenotypes during tissue repair. J Leukoc Biol. 2013;93(6):875-881. [21] ZHU Z, DING J, MA Z, et al. The natural behavior of mononuclear phagocytes in HTS formation. Wound Repair Regen. 2016;24(1):14-25. [22] HSU YC, CHEN MJ, YU YM, et al. Suppression of TGF-β1/SMAD pathway and extracellular matrix production in primary keloid fibroblasts by curcuminoids: its potential therapeutic use in the chemoprevention of keloid. Arch Dermatol Res. 2010;302(10):717-724. [23] CHEN J, ZENG B, YAO H, et al. The effect of TLR4/7 on the TGF-β-induced Smad signal transduction pathway in human keloid. Burns. 2013;39(3):465-472. [24] SHEN D, HE Z. Mesenchymal stem cell-derived exosomes regulate the polarization and inflammatory response of macrophages via miR-21-5p to promote repair after myocardial reperfusion injury. Ann Transl Med. 2021;9(16):1323. [25] TI D, HAO H, TONG C, et al. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J Transl Med. 2015;13:308. [26] MORADI-CHALESHTORI M, BANDEHPOUR M, HEIDARI N, et al. Exosome-mediated miR-33 transfer induces M1 polarization in mouse macrophages and exerts antitumor effect in 4T1 breast cancer cell line. Int Immunopharmacol. 2021;90:107198. [27] BERSCHNEIDER B, ELLWANGER DC, BAARSMA HA, et al. miR-92a regulates TGF-β1-induced WISP1 expression in pulmonary fibrosis. Int J Biochem Cell Biol. 2014;53:432-441. [28] CHAE DK, BAN E, YOO YS, et al. MIR-27a regulates the TGF-β signaling pathway by targeting SMAD2 and SMAD4 in lung cancer. Mol Carcinog. 2017;56(8):1992-1998. [29] DING F, YOU T, HOU XD, et al. MiR-21 regulates pulmonary hypertension in rats via TGF-β1/Smad2 signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(9): 3984-3992. [30] JAHANGIRIMOEZ M, MEDLEJ A, TAVALLAIE M, et al. Hsa-miR-587 Regulates TGFβ/SMAD Signaling and Promotes Cell Cycle Progression. Cell J. 2020;22(2):158-164. [31] ZHAO S, NING Y, QIN N, et al. GAS5 regulates viability and apoptosis in TGF-β1-stimulated bronchial epithelial cells by regulating miR-217/HDAC4 axis. Genes Genomics. 2021;43(8):837-846. [32] JIN J, JIA ZH, LUO XH, et al. Long non-coding RNA HOXA11-AS accelerates the progression of keloid formation via miR-124-3p/TGFβR1 axis. Cell Cycle. 2020; 19(2):218-232. [33] 罗雅婷,解婧,许涛,等.人脐带间充质干细胞来源外泌体对小鼠压疮的治疗作用及机制[J].海军军医大学学报,2022,43(6):622-632. [34] YUAN R, DAI X, LI Y, et al. Exosomes from miR-29a-modified adipose-derived mesenchymal stem cells reduce excessive scar formation by inhibiting TGF-β2/Smad3 signaling. Mol Med Rep. 2021;24(5):758. [35] LI X, LIU L, YANG J, et al. Exosome Derived From Human Umbilical Cord Mesenchymal Stem Cell Mediates MiR-181c Attenuating Burn-induced Excessive Inflammation. EBioMedicine. 2016;8:72-82. [36] XIE J, WU W, ZHENG L, et al. Roles of MicroRNA-21 in Skin Wound Healing: A Comprehensive Review. Front Pharmacol. 2022;13:828627. [37] AL-ATTAR A, MESS S, THOMASSEN JM, et al. Keloid pathogenesis and treatment. Plast Reconstr Surg. 2006;117(1):286-300. [38] VERHAEGEN PD, VAN ZUIJLEN PP, PENNINGS NM, et al. Differences in collagen architecture between keloid, hypertrophic scar, normotrophic scar, and normal skin: An objective histopathological analysis. Wound Repair Regen. 2009;17(5): 649-656. [39] SCHULTZ GS, DAVIDSON JM, KIRSNER RS, et al. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen. 2011;19(2):134-148. [40] LEE DE, TROWBRIDGE RM, AYOUB NT, et al. High-mobility Group Box Protein-1, Matrix Metalloproteinases, and Vitamin D in Keloids and Hypertrophic Scars. Plast Reconstr Surg Glob Open. 2015;3(6):e425. [41] ZHAO X, KWAN JYY, YIP K, et al. Targeting metabolic dysregulation for fibrosis therapy. Nat Rev Drug Discov. 2020;19(1):57-75. [42] MCKLEROY W, LEE TH, ATABAI K. Always cleave up your mess: targeting collagen degradation to treat tissue fibrosis. Am J Physiol Lung Cell Mol Physiol. 2013;304(11):L709-721. [43] CALEY MP, MARTINS VL, O’TOOLE EA. Metalloproteinases and Wound Healing. Adv Wound Care (New Rochelle). 2015;4(4):225-234. [44] 李文娟,陈伟,王海滨,等.不同发生时期的增生性瘢痕中基质金属蛋白酶及其抑制因子基因表达的变化[J].感染、炎症、修复,2006,7(1):14-17. [45] FUJIWARA M, MURAGAKI Y, OOSHIMA A. Keloid-derived fibroblasts show increased secretion of factors involved in collagen turnover and depend on matrix metalloproteinase for migration. Br J Dermatol. 2005;153(2):295-300. [46] GHAHARY A, GHAFFARI A. Role of keratinocyte-fibroblast cross-talk in development of hypertrophic scar. Wound Repair Regen. 2007;15 Suppl 1:S46-53. [47] RUSSO B, BREMBILLA NC, CHIZZOLINI C. Interplay Between Keratinocytes and Fibroblasts: A Systematic Review Providing a New Angle for Understanding Skin Fibrotic Disorders. Front Immunol. 2020;11:648. [48] NOWINSKI D, LYSHEDEN AS, GARDNER H, et al. Analysis of gene expression in fibroblasts in response to keratinocyte-derived factors in vitro: potential implications for the wound healing process. J Invest Dermatol. 2004;122(1):216-221. [49] FUNAYAMA E, CHODON T, OYAMA A, et al. Keratinocytes promote proliferation and inhibit apoptosis of the underlying fibroblasts: an important role in the pathogenesis of keloid. J Invest Dermatol. 2003;121(6):1326-1331. [50] MA T, FU B, YANG X, et al. Adipose mesenchymal stem cell-derived exosomes promote cell proliferation, migration, and inhibit cell apoptosis via Wnt/β-catenin signaling in cutaneous wound healing. J Cell Biochem. 2019;120(6): 10847-10854. [51] YAN L, CAO R, LIU Y, et al. MiR-21-5p Links Epithelial-Mesenchymal Transition Phenotype with Stem-Like Cell Signatures via AKT Signaling in Keloid Keratinocytes. Sci Rep. 2016;6:28281. [52] AZEVEDO ML, SILVEIRA RG, NEDEL F, et al. MicroRNAs expressed during normal wound healing and their associated pathways: A systematic review and bioinformatics analysis. PLoS One. 2023;18(4):e0281913. [53] ZAKERI A, KHASEB S, AKHAVAN RAHNAMA M, et al. Exosomes derived from mesenchymal stem cells: A promising cell-free therapeutic tool for cutaneous wound healing. Biochimie. 2023;209:73-84. [54] YANG C, LUO L, BAI X, et al. Highly-expressed micoRNA-21 in adipose derived stem cell exosomes can enhance the migration and proliferation of the HaCaT cells by increasing the MMP-9 expression through the PI3K/AKT pathway. Arch Biochem Biophys. 2020;681:108259. [55] ZHU G, YANG X, PENG C, et al. Exosomal miR-532-5p from bone marrow mesenchymal stem cells reduce intervertebral disc degeneration by targeting RASSF5. Exp Cell Res. 2020;393(2):112109. [56] WU D, LIU X, JIN Z. Adipose‐derived mesenchymal stem cells‐sourced exosomal microRNA‐7846‐3p suppresses proliferation and pro‐angiogenic role of keloid fibroblasts by suppressing neuropilin 2. J Cosmet Dermatol. 2023;22(8):2333-2342. [57] ZHAO W, ZHANG H, LIU R, et al. Advances in Immunomodulatory Mechanisms of Mesenchymal Stem Cells-Derived Exosome on Immune Cells in Scar Formation. Int J Nanomedicine. 2023;18:3643-3662. [58] YAN L, WANG LZ, XIAO R, et al. Inhibition of microRNA-21-5p reduces keloid fibroblast autophagy and migration by targeting PTEN after electron beam irradiation. Lab Invest. 2020;100(3):387-399. [59] LI XM, YU WY, CHEN Q, et al. LncRNA TUG1 exhibits pro-fibrosis activity in hypertrophic scar through TAK1/YAP/TAZ pathway via miR-27b-3p. Mol Cell Biochem. 2021;476(8):3009-3020. [60] XU M, SUN J, YU Y, et al. TM4SF1 involves in miR-1-3p/miR-214-5p-mediated inhibition of the migration and proliferation in keloid by regulating AKT/ERK signaling. Life Sci. 2020;254:117746. [61] YAO Q, XING Y, WANG Z, et al. MiR-16-5p suppresses myofibroblast activation in systemic sclerosis by inhibiting NOTCH signaling. Aging (Albany NY). 2020; 13(2):2640-2654. [62] YANG J, DENG P, QI Y, et al. NEAT1 Knockdown Inhibits Keloid Fibroblast Progression by miR-196b-5p/FGF2 Axis. J Surg Res. 2021;259:261-270. [63] LI Y, ZHANG J, SHI J, et al. Exosomes derived from human adipose mesenchymal stem cells attenuate hypertrophic scar fibrosis by miR-192-5p/IL-17RA/Smad axis. Stem Cell Res Ther. 2021;12(1):221. [64] CHEN J, YU W, XIAO C, et al. Exosome from adipose-derived mesenchymal stem cells attenuates scar formation through microRNA-181a/SIRT1 axis. Arch Biochem Biophys. 2023;746:109733. [65] ZHANG M, CHEN H, QIAN H, et al. Characterization of the skin keloid microenvironment. Cell Commun Signal. 2023;21(1):207. [66] MCGARRY T, BINIECKA M, VEALE DJ, et al. Hypoxia, oxidative stress and inflammation. Free Radic Biol Med. 2018;125:15-24. [67] HONG L, JUNJIE C, PENGYU Z, et al. The mechanism of oxidative stress in keloid fibroblasts and the experimental study of early application of angiotensin-converting enzyme inhibitor. Indian J Dermatol Venereol Leprol. 2023:1-8. doi: 10.25259/IJDVL_323_2022. Epub ahead of print. [68] WANG Q, YANG X, MA J, et al. PI3K/AKT pathway promotes keloid fibroblasts proliferation by enhancing glycolysis under hypoxia. Wound Repair Regen. 2023; 31(2):139-155. [69] QIN H, ZHANG L, LI M, et al. EGR1/NOX4 pathway regulates oxidative stress and further facilitates fibrosis progression in keloids responses to TGF-β1. J Dermatol Sci. 2022;108(3):138-145. [70] WU H, WANG J, MA H, et al. MicroRNA-21 inhibits mitochondria-mediated apoptosis in keloid. Oncotarget. 2017;8(54):92914-92925. [71] ZHANG J, XU D, LI N, et al. Downregulation of microRNA-31 inhibits proliferation and induces apoptosis by targeting HIF1AN in human keloid. Oncotarget. 2017; 8(43):74623-74634. [72] LV W, REN Y, WU M, et al. Identifying miRNA modules associated with progression of keloids through weighted gene co-expression network analysis and experimental validation in vitro. Burns. 2021;47(6):1359-1372. [73] HE Y, ZHANG Z, YIN B, et al. Identifying miRNAs Associated with the Progression of Keloid through mRNA-miRNA Network Analysis and Validating the Targets of miR-29a-3p in Keloid Fibroblasts. Biomed Res Int. 2022;2022:6487989. [74] POLTAVTSEVA RA, POLTAVTSEV AV, LUTSENKO GV, et al. Myths, reality and future of mesenchymal stem cell therapy. Cell Tissue Res. 2019;375(3):563-574. |
| [1] | Chen Kaijia, Liu Jingyun, Cao Ning, Sun Jianbo, Zhou Yan, Mei Jianguo, Ren Qiang. Application and prospect of tissue engineering in treatment of osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1450-1456. |
| [2] | Yang Yifeng, Ye Nan, Wang Lin, Guo Shuaicheng, Huang Jian. Signaling pathway of dexmedetomidine against ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1464-1469. |
| [3] | Shen Jiangyong, He Xi, Tang Yuting, Wang Jianjun, Liu Jinyi, Chen Yuanyuan, Wang Xinyi, Liu Tong, Sun Haoyuan. RAS-selective lethal small molecule 3 inhibits the fibrosis of pathological scar fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1168-1173. |
| [4] | Wang Ji, Zhang Min, Li Wenbo, Yang Zhongya, Zhang Long. Effect of aerobic exercise on glycolipid metabolism, skeletal muscle inflammation and autophagy in type 2 diabetic rats [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1200-1205. |
| [5] | Lou Guo, Zhang Yan, Fu Changxi. Role of endothelial nitric oxide synthase in exercise preconditioning-induced improvement of myocardial ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1283-1288. |
| [6] | Wang Weiqing, Zhou Yue. Chronic inflammation regulates adipose tissue fibrosis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1307-1312. |
| [7] | Mu Bingtao, Yu Jingwen, Liu Chunyun, Guo Minfang, Meng Tao, Yang Pengwei, Wei Wenyue, Song Lijuan, Yu Jiezhong, Ma Cungen. Immunomodulatory effect of astragaloside IV on T cells of experimental autoimmune encephalomyelitis mice [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1057-1062. |
| [8] | Liu Jianhong, Liao Shijie, Li Boxiang, Tang Shengping, Wei Zhendi, Ding Xiaofei. Extracellular vesicles carrying non-coding RNA regulate the activation of osteoclasts [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1076-1082. |
| [9] | Pan Xiaolong, Fan Feiyan, Ying Chunmiao, Liu Feixiang, Zhang Yunke. Effect and mechanism of traditional Chinese medicine on inhibiting the aging of mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1091-1098. |
| [10] | Liu Hanfeng, Wang Jingjing, Yu Yunsheng. Artificial exosomes in treatment of myocardial infarction: current status and prospects [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1118-1123. |
| [11] | Liu Tao, He Zhijun, Li Jinpeng, Song Yuan, Yao Xingzhang, Chen Wen, Li Yan, Bai Bihui. Role and mechanism of noncoding RNA in diabetic peripheral neuropathy [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1124-1129. |
| [12] | Ma Shuwei, He Sheng, Han Bing, Zhang Liaoyun. Exosomes derived from mesenchymal stem cells in treatment of animals with acute liver failure: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1137-1142. |
| [13] | Feng Ruiqin, Han Na, Zhang Meng, Gu Xinyi, Zhang Fengshi. Combination of 1% platelet-rich plasma and bone marrow mesenchymal stem cells improves the recovery of peripheral nerve injury [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 985-992. |
| [14] | Qiu Xiaoyan, Li Bixin, Li Jingdi, Fan Chuiqin, Ma Lian, Wang Hongwu. Differentiation of insulin-producing cells from human umbilical cord mesenchymal stem cells infected by MAFA-PDX1 overexpressed lentivirus [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1000-1006. |
| [15] | Liu Qiwei, Zhang Junhui, Yang Yuan, Wang Jinjuan. Role and mechanism of umbilical cord mesenchymal stem cells on polycystic ovary syndrome [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1015-1020. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||