Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (31): 5022-5028.doi: 10.12307/2024.726
Previous Articles Next Articles
Astragaloside inhibits astrocyte activation and inflammatory response induced by inflammation
Yu Jingwen1, Guo Minfang1, Zhang Bingxin1, Mu Bingtao1, Meng Tao1, Zhang Huiyu1, Ma Cungen1, 2, Yin Jinzhu3, Song Lijuan2, 3, Yu Jiezhong1, 2, 4
- 1Institute of Brain Science/Key Laboratory of Molecular Cellular Immunology in Datong City/Department of Neurology of First Affiliated Hospital, Shanxi Datong University, Datong 037009, Shanxi Province, China; 2Key Research Laboratory of Benefiting Qi for Acting Blood Circulation Method to Treat Multiple Sclerosis of State Administration of Traditional Chinese Medicine/Research Center of Neurobiology, Shanxi University of Chinese Medicine, Jinzhong 030619, Shanxi Province, China;
-
Received:2023-09-06Accepted:2023-11-01Online:2024-11-08Published:2024-01-22 -
Contact:Yu Jiezhong, MD, Professor, Institute of Brain Science/Key Laboratory of Molecular Cellular Immunology in Datong City/Department of Neurology of First Affiliated Hospital, Shanxi Datong University, Datong 037009, Shanxi Province, China; Key Research Laboratory of Benefiting Qi for Acting Blood Circulation Method to Treat Multiple Sclerosis of State Administration of Traditional Chinese Medicine/Research Center of Neurobiology, Shanxi University of Chinese Medicine, Jinzhong 030619, Shanxi Province, China; The Fifth People’s Hospital of Datong, Datong 037009, Shanxi Province, China Song Lijuan, MD, Associate professor, Key Research Laboratory of Benefiting Qi for Acting Blood Circulation Method to Treat Multiple Sclerosis of State Administration of Traditional Chinese Medicine/Research Center of Neurobiology, Shanxi University of Chinese Medicine, Jinzhong 030619, Shanxi Province, China; Department of Neurosurgery, Tongmei General Hospital/Key Laboratory of Prevention and Treatment of Neurological Diseases, Shanxi Provincial Health Commission, Datong 037003, Shanxi Province, China -
About author:Yu Jingwen, Master, Senior experimentalist, Institute of Brain Science/Key Laboratory of Molecular Cellular Immunology in Datong City/Department of Neurology of First Affiliated Hospital, Shanxi Datong University, Datong 037009, Shanxi Province, China -
Supported by:Shanxi Basic Research Program, No. 20210302123337 (to YJW); Shanxi Basic Research Program, No. 20210302123478 (to ZHY); University Level Research Project of Shanxi Datong University, No. 2022K17 (to YJW); Innovation and Entrepreneurship Training Program of Shanxi College Students, No. XDC2022119 (to ZBX); Medical Science and Technology Leading Team of Shanxi Provincial Health Commission, No. 2020TD05 (to MCG); Zhang Zhongjing Inheritance and Innovation Project of National Administration of Traditional Chinese Medicine, No. GDY-KJS-2022-048-1 (to SLJ); 2022 Shanxi Science and Technology Innovation Young Talent Team, No. 202204051001028 (to SLJ); 2022 Traditional Chinese Medicine Research Project Plan of Shanxi Provincial Health Commission, No. 2022ZYYC090 (to MCG); Young Scientist Training Project of Shanxi University of Chinese Medicine, No. 2021-PY-QN-09 (to SLJ); 2022 Annual Science and Technology Innovation Team of Shanxi University of Chinese Medicine, No. 2022TD2010 (to MCG, SLJ)
CLC Number:
Cite this article
Yu Jingwen, Guo Minfang, Zhang Bingxin, Mu Bingtao, Meng Tao, Zhang Huiyu, Ma Cungen, Yin Jinzhu, Song Lijuan, Yu Jiezhong. Astragaloside inhibits astrocyte activation and inflammatory response induced by inflammation[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(31): 5022-5028.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
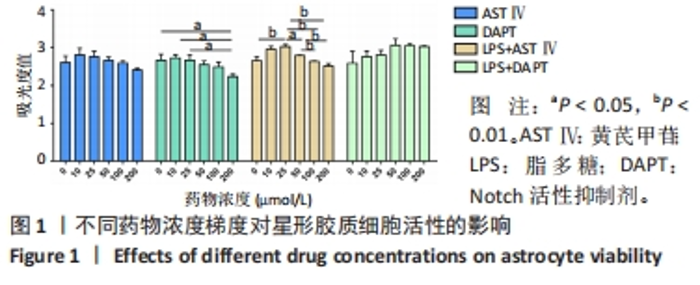
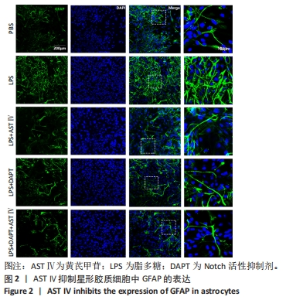
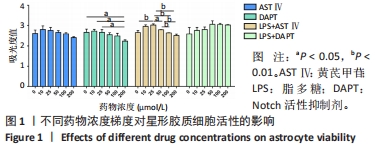
2.1 不同处理方法对星形胶质细胞活性的影响 从图1结果可知,AST Ⅳ单独作用于星形胶质细胞时(即AST Ⅳ组),在10-100 μmol/L范围内细胞活力受AST Ⅳ影响较小,与对照组相比差异不显著;DAPT单独作用于星形胶质细胞时(即DAPT组),10,25 μmol/L DAPT对细胞活力影响较小,与对照组相比差异不显著,当浓度达到200 μmol/L时细胞活力与对照组和低剂量(10,25 μmol/L)相比差异有显著性意义(均P < 0.05);LPS+AST Ⅳ作用于星形胶质细胞时(即LPS+AST Ⅳ组),AST Ⅳ浓度在25 μmol/L时与对照组(只加1 mg/mL LPS,不加AST Ⅳ)相比,细胞活力显著增加(P < 0.01),当AST Ⅳ浓度在50-200 μmol/L时,细胞活力与25 μmol/L相比,则明显下降(P < 0.05,P < 0.01);LPS+DAPT作用于星形胶质细胞时(即LPS+DAPT组),DAPT浓度在10-200 μmol/L时,细胞活力有增加的趋势,但无统计学意义,浓度在50 μmol/L时,细胞活力高于其他组。故该实验选取AST Ⅳ浓度为25 μmol/L,DAPT浓度为50 μmol/L。 "
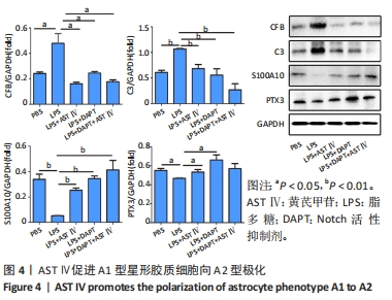
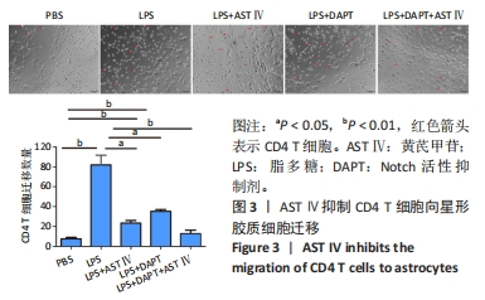
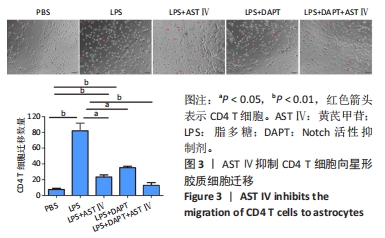
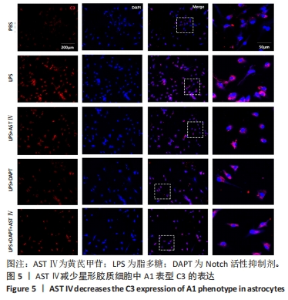
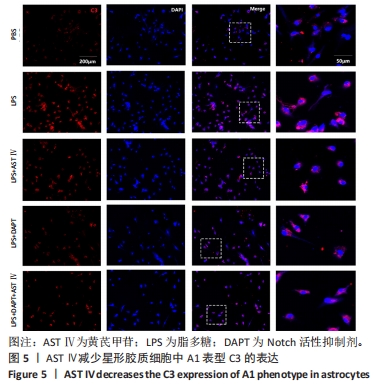
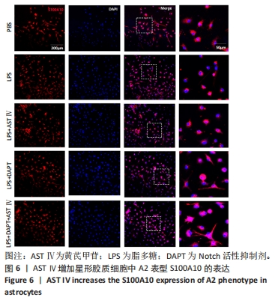
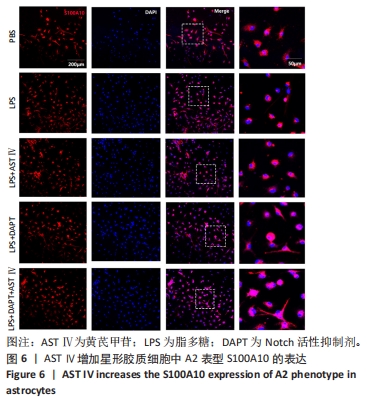
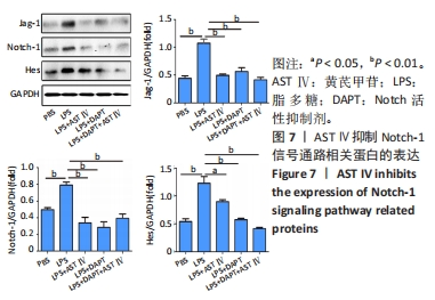
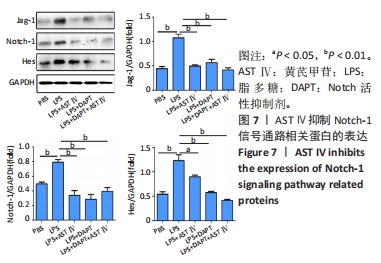
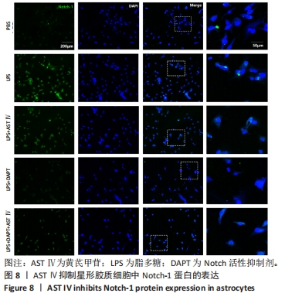
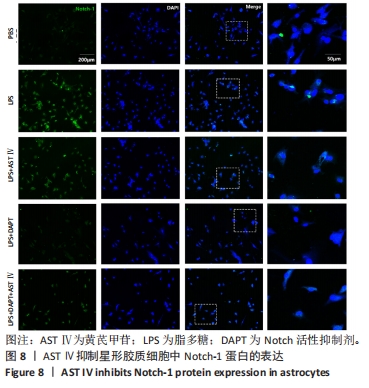
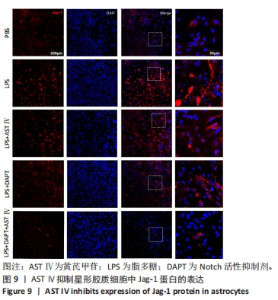
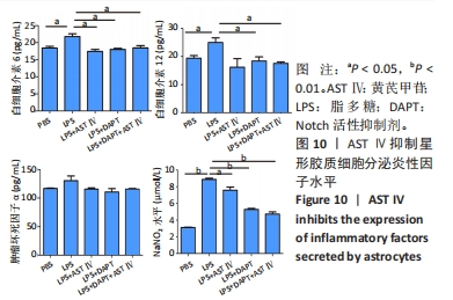
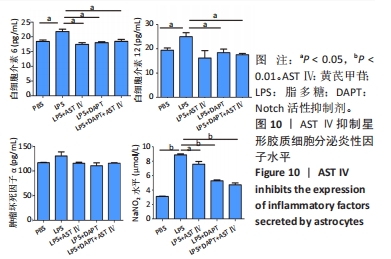
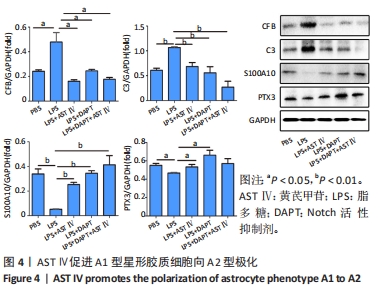
2.4 AST Ⅳ促进A1型星形胶质细胞向A2型极化 利用Western blot法检测5组细胞A1型标记物CFB,C3以及A2型标记物S100A10,PTX3的表达。由图4可见,与PBS组比较,LPS组CFB和C3表达明显增加(P < 0.05,P < 0.01);与LPS组比较,LPS+AST Ⅳ组、LPS+DAPT组和LPS+DAPT+AST Ⅳ组CFB(均P < 0.05)和C3(均P < 0.01)表达明显减少。与PBS组比较,LPS组S100A10和PTX3表达减少(P < 0.01,P < 0.05);与LPS组比较,LPS+AST Ⅳ组、LPS+DAPT组和LPS+DAPT+AST Ⅳ组S100A10(均P < 0.01)和PTX3(P < 0.05,P < 0.05,P > 0.05)表达明显增加。"
| [1] VERKHRATSKY A, HO MS, PARPURA V. Evolution of Neuroglia. Adv Exp Med Biol. 2019;1175: 15-44. [2] WU Q, MIAO X, ZHANG J, et al. Astrocytic YAP protects the optic nerve and retina in an experimental autoimmune encephalomyelitis model through TGF-β signaling. Theranostics. 2021;11(17):8480-8499. [3] CHUN H, IM H, KANG YJ, et al. Severe reactive astrocytes precipitate pathological hallmarks of Alzheimer’s disease via H2O2- production. Nat Neurosci. 2020;23(12):1555-1566. [4] WILLIAMSON MR, FUERTES CJA, DUNN AK, et al. Reactive astrocytes facilitate vascular repair and remodeling after stroke. Cell Rep. 2021;35(4):109048. [5] ZHOU B, ZUO YX, JIANG RT. Astrocyte morphology: Diversity, plasticity, and role in neurological diseases. CNS Neurosci Ther. 2019;25(6):665-673. [6] WHEELER MA, QUINTANA FJ. Regulation of Astrocyte Functions in Multiple Sclerosis. Cold Spring Harb Perspect Med. 2019;9(1):a029009. [7] RUPARELIYA VP, SINGH AA, BUTT AM, et al. The “molecular soldiers” of the CNS: Astrocytes, a comprehensive review on their roles and molecular signatures. Eur J Pharmacol. 202;959:176048. [8] QIAN D, LI L, RONG Y, et al. Blocking Notch signal pathway suppresses the activation of neurotoxic A1 astrocytes after spinal cord injury. Cell Cycle. 2019;18(21):3010-3029. [9] OHUCHI K, FUNATO M, YOSHINO Y, et al. Notch Signaling Mediates Astrocyte Abnormality in Spinal Muscular Atrophy Model Systems. Sci Rep. 2019;9(1):3701. [10] LI QQ, DING DH, WANG XY, et al. Lipoxin A4 regulates microglial M1/M2 polarization after cerebral ischemia-reperfusion injury via the Notch signaling pathway. Exp Neurol. 2021;339:113645. [11] 孙洁.黄芪及其经方的临床应用研究进展[J].中国医院药学志,2019,39(12):1311-1314. [12] 于婧文,郭敏芳,张婧,等.黄芪甲苷对体外由脂多糖诱导的星形胶质 细胞炎性反应及其作用机制的研究[J].中华微生物学和免疫学杂志,2018,38(11):829-834. [13] LIDDELOW SA, GUTTENPLAN KA, CLARKE LE, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481-487. [14] KWON HS, KOH SH. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener. 2020;9(1):42. [15] LINNERBAUER M, WHEELER MA, QUINTANA FJ. Astrocyte Crosstalk in CNS Inflammation. Neuron. 2020;108(4):608-622. [16] PONATH G, PARK C, PITT D. The Role of Astrocytes in Multiple Sclerosis.Front Immunol. 2018;9:217. [17] BRAMBILLA R. The contribution of astrocytes to the neuroinflammatory response in multiple sclerosis and experimental autoimmune encephalomyelitis. Acta Neuropathol. 2019;137(5):757-783. [18] STÜVE O, YOUSSEF S, SLAVIN AJ, et al. The role of the MHC class II transactivator in class II expression and antigen presentation by astrocytes and in susceptibility to central nervous system autoimmune disease. J Immunol. 2002;169(12):6720-6732. [19] LI Z, HAN J, REN H, et al. Astrocytic Interleukin-15 Reduces Pathology of Neuromyelitis Optica in Mice. Front Immunol. 2018;9:523. [20] 穆秉桃,于婧文,刘春云,等.黄芪甲苷对实验性自身免疫性脑脊髓炎小鼠T细胞免疫调节的影响[J].中国组织工程研究,2024,28(7):1057-1062. [21] YU J, MU B, GUO M, et al. Astragaloside IV inhibits experimental autoimmune encephalomyelitis by modulating the polarization of both microglia/macrophages and astrocytes. Folia Neuropathol. 2023;61(3):273-290. [22] LIU X, ZHOU F, YANG Y, et al. MiR-409-3p and MiR-1896 co-operatively participate in IL-17-induced inflammatory cytokine production in astrocytes and pathogenesis of EAE mice via targeting SOCS3/STAT3 signaling. Glia. 2019;67(1):101-112. [23] ROSTAMI J, FOTAKI G, SIROIS J, et al. Astrocytes have the capacity to act as antigen-presenting cells in the Parkinson’s disease brain. J Neuroinflammation. 2020;17(1):119. [24] CORNET A, BETTELLI E, OUKKA M, et al. Role of astrocytes in antigen presentation and naive T-cell activation. J Neuroimmunol. 2000;106(1-2):69-77. [25] HAMO L, STOHLMAN SA, OTTO-DUESSEL M, et al. Distinct regulation of MHC molecule expression on astrocytes and microglia during viral encephalomyelitis. Glia. 2007; 55(11):1169-1177. [26] GUO MF, ZHANG HY, LI YH, et al. Fasudil inhibits the activation of microglia and astrocytes of transgenic Alzheimer’s disease mice via the downregulation of TLR4/Myd88/NF-κB pathway. J Neuroimmunol. 2020;346:577284. [27] XU J, HU C, CHEN S, et al. Neuregulin-1 protects mouse cerebellum against oxidative stress and neuroinflammation. Brain Res. 2017;1670:32-43. [28] HOU B, ZHANG Y, LIANG P, et al. Inhibition of the NLRP3-inflammasome prevents cognitive deficits in experimental autoimmune encephalomyelitis mice via the alteration of astrocyte phenotype. Cell Death Dis. 2020;11(5):377. [29] CEYZÉRIAT K, BEN HAIM L, DENIZOT A, et al. Modulation of astrocyte reactivity improves functional deficits in mouse models of Alzheimer’s disease. Acta Neuropathol Commun. 2018;6(1):104. [30] ZHAO YL, YAO HJ, LI ZG, et al. Influence of electroacupuncture of “Dazhui”(GV14) and “Mingmen” (GV4) on expressions of NL1 and NF-200 in spinal cord injury rats. Zhen Ci Yan Jiu. 2023;48(9):906-913. [31] MARUMO T, TAKAGI Y, MURAKI K, et al. Notch signaling regulates nucleocytoplasmic Olig2 translocation in reactive astrocytes differentiation after ischemic stroke. Neurosci Res. 2013;75(3):204-209. [32] LI DY, GAO SJ, SUN J, et al. Notch signaling activation contributes to paclitaxel-induced neuropathic pain via activation of A1 astrocytes. Eur J Pharmacol. 2022;928:175130. [33] FAN SR, REN TT, YUN MY, et al. Edaravone attenuates cadmium-induced toxicity by inhibiting oxidative stress and inflammation in ICR mice. Neurotoxicology. 202;86:1-9. |
| [1] | Mu Bingtao, Yu Jingwen, Liu Chunyun, Guo Minfang, Meng Tao, Yang Pengwei, Wei Wenyue, Song Lijuan, Yu Jiezhong, Ma Cungen. Immunomodulatory effect of astragaloside IV on T cells of experimental autoimmune encephalomyelitis mice [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1057-1062. |
| [2] | Liu Kexin, Song Lijuan, Wu Yige, Han Guangyuan, Miao Zhuyue, Wei Ruheng, Xiao Baoguo, Ma Cungen, Huang Jianjun. Hydroxysafflor yellow A intervenes astrocyte lipocalin 2 expression after cerebral ischemia/reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(7): 1063-1069. |
| [3] | Li Zhen, Sun Xiao, Xie Yongpeng, Rong Wang, Sun Haitao. Neuron-derived extracellular vesicles promote neurogenesis of neural stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(25): 3994-3999. |
| [4] | Ye Jiapeng, Wang Jianwei, Wu Mao, Li Shaoshuo, Wang Guopeng, Wang Haotian, Tang Zhi, Shao Yang. miR-146a-3p regulates astrocyte proliferation, migration and apoptosis by inhibiting insulin-like growth factor 1 expression [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(25): 4048-4053. |
| [5] | Han Yue, Wang Yufei, Liu Wanqing, Dong Ming, Niu Weidong. Effects of icariin on proliferation and differentiation of MC3T3-E1 cells in an inflammatory environment [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(23): 3709-3714. |
| [6] | Yang Qihang, Pu Rui, Chen Ziyang, Leng Siyi, Song Yongjing, Liu Hui, Du Guangyou. Role and mechanism of intestinal flora metabolites in obesity regulation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(2): 308-314. |
| [7] | Chen Na, Wang Yanlin, Sun Huifang, Fan Feiyan, Li Donghong, Zhang Yunke. Shexiang Huangqi compound dripping pills-containing serum promotes proliferation and differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(19): 2960-2966. |
| [8] | Yang Runze, Wang Wei, Chen San, Zhou Xuedong, Wu Jiayuan. Effect of exosomes and the preconditioning method on pulp regeneration [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(13): 2105-2113. |
| [9] | Bai Zhengrong, Sun Yu, Zhang Zhenxian, Pan Shinong. Delayed onset muscle soreness and exercise-induced skeletal muscle memory [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(11): 1762-1766. |
| [10] | Yang Ting, Ding Zhibin, Jiang Nan, Han Hongxia, Hou Miaomiao, Ma Cungen, Song Lijuan, Li Xinyi. Astrocytes regulate glial scar formation in cerebral ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(1): 131-138. |
| [11] | Long Qingxi, Zhang Pingshu, Liu Qing, Ou Ya, Zhang Lili, Yuan Xiaodong. Single-cell RNA sequencing reveals the heterogeneity of astrocytes [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(1): 139-146. |
| [12] | Gao Yu, Han Jiahui, Ge Xin. Immunoinflammatory microenvironment after spinal cord ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(8): 1300-1305. |
| [13] | Hao Liufang, Duan Hongmei, Wang Zijue, Hao Fei, Hao Peng, Zhao Wen, Gao Yudan, Yang Zhaoyang, Li Xiaoguang. Spatiotemporal dynamic changes of ependymal cells after spinal cord injury in transgenic mice [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(6): 883-889. |
| [14] | Chen Guodong, Zheng Meiyan, Zhang Peng, Wang Zhenchao, Jin Lixin. Changes in sensory neurons and astrocytes and the expression of interleukin 1beta and glial fibrillary acidic protein in the rat spinal cord after selective dorsal rhizotomy [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(5): 726-731. |
| [15] | Qin Yixiong, Yuan Zijian, Xie Shanzhou, Zhu Yingwen, Chen Minghui, Han Biao, Guo Yong. Effect of tea polyphenols on proliferation and differentiation of osteoblasts stimulated by lipopolysaccharide [J]. Chinese Journal of Tissue Engineering Research, 2023, 27(35): 5665-5669. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||