Chinese Journal of Tissue Engineering Research ›› 2024, Vol. 28 ›› Issue (29): 4612-4619.doi: 10.12307/2024.530
Previous Articles Next Articles
Fe-Co/ZIF-8@SLC-0111-HA composite nanoplatform enhances feasibility of tumor chemodynamic therapy
Wang Zhenxin1, Zhou Peng2, Chu Fuchao1, Zhang Dazhen1, Yuan Feng3
- 1First Clinical Medical College of Xuzhou Medical University, Xuzhou 221006, Jiangsu Province, China; 2Department of Orthopedics, Affiliated Huai’an Hospital of Xuzhou Medical University, Huai’an Second People’s Hospital, Huai’an 223002, Jiangsu Province, China; 3Department of Orthopedics, Affiliated Hospital of Xuzhou Medical University, Xuzhou 221006, Jiangsu Province, China
-
Received:2023-09-22Accepted:2023-11-04Online:2024-10-18Published:2024-03-22 -
Contact:Yuan Feng, Professor, MD, Department of Orthopedics, Affiliated Hospital of Xuzhou Medical University, Xuzhou 221006, Jiangsu Province, China -
About author:Wang Zhenxin, Master candidate, First Clinical Medical College of Xuzhou Medical University, Xuzhou 221006, Jiangsu Province, China -
Supported by:Key Project of Jiangsu Provincial Health Commission Medical Research, No. ZD2022064 (to YF); Jiangsu Province Traditional Chinese Medicine Technology Development Plan, No. MS2021102 (to YF); Key Laboratory Construction Project for Bone Tissue Regeneration and Digital Medicine, No. PT0306 (to YF); Jiangsu Province Graduate Research Innovation Program Project, No. KYCX23_2982 (to WZX)
CLC Number:
Cite this article
Wang Zhenxin, Zhou Peng, Chu Fuchao, Zhang Dazhen, Yuan Feng. Fe-Co/ZIF-8@SLC-0111-HA composite nanoplatform enhances feasibility of tumor chemodynamic therapy[J]. Chinese Journal of Tissue Engineering Research, 2024, 28(29): 4612-4619.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
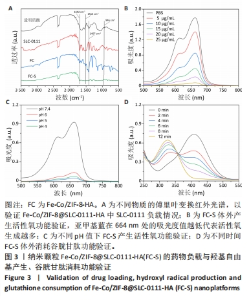
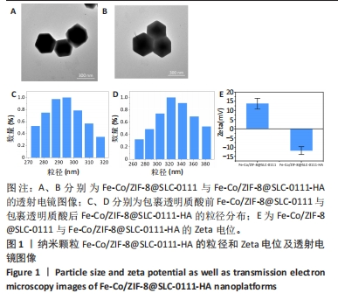
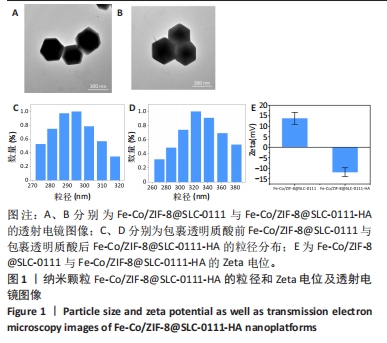
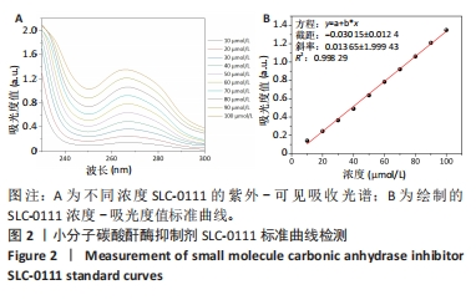
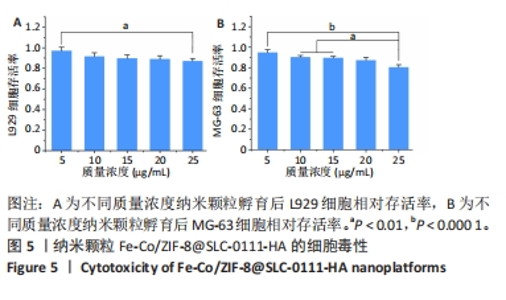
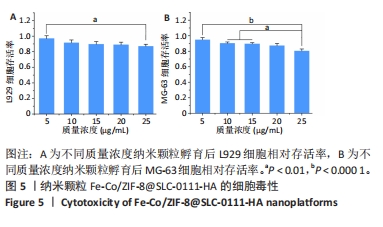
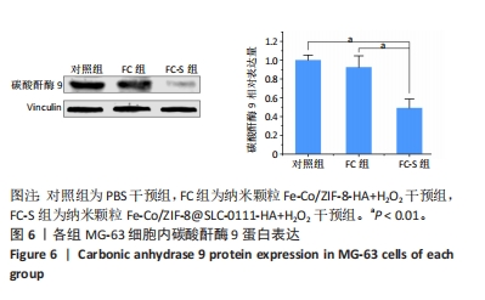
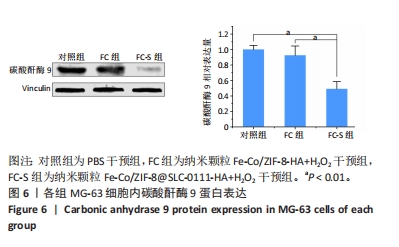
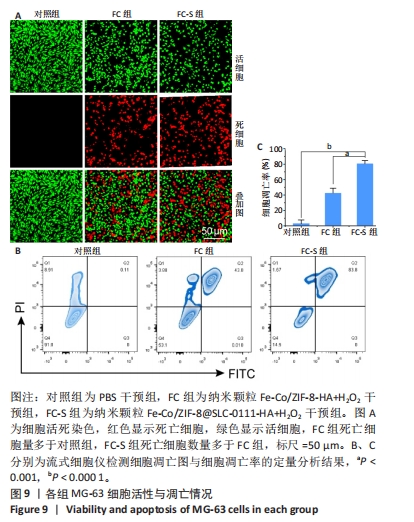
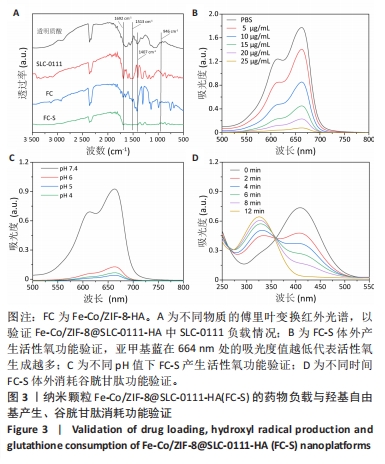
2.1.3 FC-S的药物负载情况 图3A是验证SLC-0111和透明质酸成功加载的结果图,在傅里叶变换红外光谱中,SLC-0111在1 692 cm-1 处出现的特征性吸收峰是羰基键伸缩振动吸收峰,而ZIF-8典型的C=N拉伸振动吸收峰在1 513 cm-1处出现,证明了Fe-Co/ZIF-8和SLC-0111的有效共存。此外在透明质酸包覆后,光谱中1 407 cm-1处和946 cm-1处分别出现明显的羧基键和糖特征吸收峰,与Zeta电位结果共同证实了透明质酸的成功包覆。 2.1.4 FC-S产生羟基自由基与消耗谷胱甘肽的能力 图3B、C是亚甲基蓝降解曲线,用于评估FC-S在PBS中产生活性氧的能力。结果显示随着FC-S质量浓度的增加和体外酸性环境的增强,吸光度值降低,说明产生的羟基自由基增加。图3D是对FC-S体外谷胱甘肽消耗功能的验证,结果表明随着时间的增加,谷胱甘肽消耗越多。"
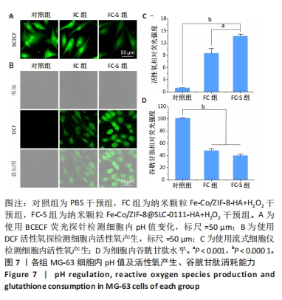
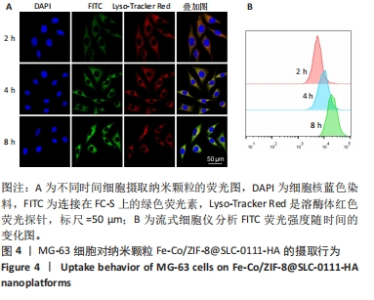
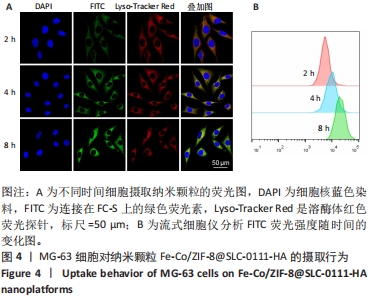
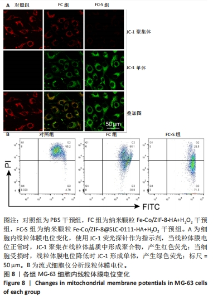
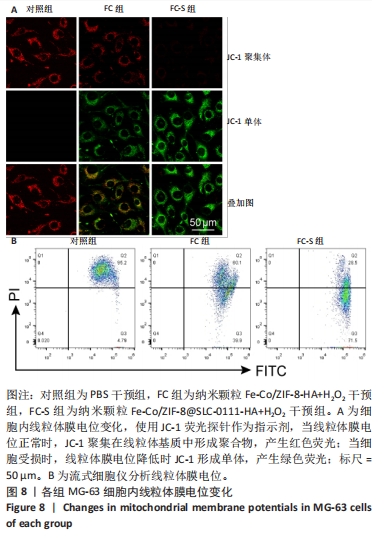
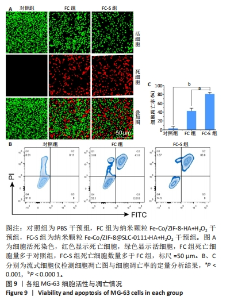
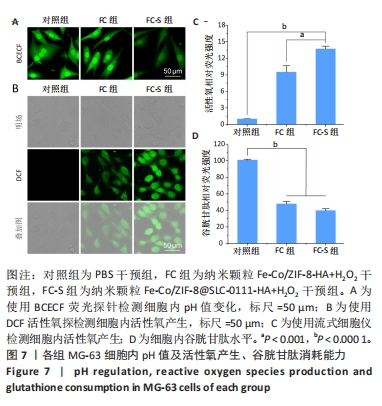
2.3.2 MG-63细胞内pH 值变化与活性氧产生 在碳酸酐酶9被成功阻断的情况下,使用BCECF指示剂进一步评估细胞内pH值变化。结果显示相较于对照组与FC组,FC-S组细胞绿色荧光减弱,表明当碳酸酐酶9活性受抑制时细胞内质子外排受阻,可有效增强细胞内酸性环境,见图7A。 采用2’,7’-二氯荧光素(DCF)作为荧光指示剂检测各组细胞内活性氧水平,评估细胞内氧化应激程度。如图7B所示,与对照组几乎没有荧光相比,FC 组呈现出中等强度的绿色荧光,这是由于铁钴双金属离子催化芬顿和类芬顿反应的发生,产生了羟基自由基;在纳米颗粒引入SLC-0111 后绿色荧光强度显著增强,显示出良好的活性氧生成能力,这主要是由于 SLC-0111 所导致细胞内酸度的增加。流式细胞仪检测显示,FC-S组绿色荧光强度高于对照组、FC 组(P < 0.001,P < 0.000 1),见图7C。图7D为不同纳米颗粒对谷胱甘肽的消耗功能,结果显示FC-S组、FC组细胞内谷胱甘肽均低于对照组(P < 0.000 1)。"
| [1] KOO S, PARK OK, KIM J, et al. Enhanced Chemodynamic Therapy by Cu-Fe Peroxide Nanoparticles: Tumor Microenvironment-Mediated Synergistic Fenton Reaction. ACS Nano. 2022;16(2):2535-2545. [2] ZHANG L, LI CX, WAN SS, et al. Nanocatalyst-Mediated Chemodynamic Tumor Therapy. Adv Healthc Mater. 2022;11(2):2101971. [3] MOHAMMED DF, MADLOOL HA, FARIS M, et al. Harnessing Inorganic Nanomaterials for Chemodynamic Cancer Therapy. Nanomedicine. 2022;17(24):1891-1906. [4] ZHAO P, LI H, BU W. A Forward Vision for Chemodynamic Therapy: Issues and Opportunities. Angew Chem Int Ed Engl. 2023;62(7): 202210415. [5] GAO H, CAO Z, LIU H, et al. Multifunctional Nanomedicines-Enabled Chemodynamic-Synergized Multimodal Tumor Therapy via Fenton and Fenton-like Reactions. Theranostics. 2023;13(6):1974-2014. [6] LI J, TIAN H, ZHU F, et al. Amorphous Ultra-Small Fe-Based Nanocluster Engineered and ICG Loaded Organo-Mesoporous Silica for GSH Depletion and Photothermal-Chemodynamic Synergistic Therapy. Adv Healthc Mater. 2022;11(21):2201986. [7] ZHUANG J, WANG B, CHEN H, et al. Efficient NIR-II Type-I AIE Photosensitizer for Mitochondria-Targeted Photodynamic Therapy through Synergistic Apoptosis-Ferroptosis. ACS Nano. 2023;17(10): 9110-9125. [8] ZHANG W, DU XF, LIU B, et al. Engineering Supramolecular Nanomedicine for Targeted Near Infrared-triggered Mitochondrial Dysfunction to Potentiate Cisplatin for Efficient Chemophototherapy. ACS Nano. 2022;16(1):1421-1435. [9] GROELLY FJ, FAWKES M, DAGG RA, et al. Targeting DNA Damage Response Pathways in Cancer. Nat Rev Cancer. 2023;23(2):78-94. [10] LI W, YIN S, SHEN Y, et al. Molecular Engineering of pH-Responsive NIR Oxazine Assemblies for Evoking Tumor Ferroptosis via Triggering Lysosomal Dysfunction. J Am Chem Soc. 2023;145(6):3736-3747. [11] YANG Z, LUO Y, HU Y, et al. Photothermo-Promoted Nanocatalysis Combined with H2S-Mediated Respiration Inhibition for Efficient Cancer Therapy. Adv Funct Mater. 2021;31:2007991. [12] ZUO W, CHEN W, LIU J, et al. Macrophage-Mimic Hollow Mesoporous Fe-Based Nanocatalysts for Self-Amplified Chemodynamic Therapy and Metastasis Inhibition via Tumor Microenvironment Remodeling. ACS Appl Mater Interfaces. 2022;14(4):5053-5065. [13] WU C, LIU Z, CHEN Z, et al. A Nonferrous Ferroptosis-Like Strategy for Antioxidant Inhibition-Synergized Nanocatalytic Tumor Therapeutics. Sci Adv. 2021;7(39):eabj8833. [14] MA Y, SU Z, ZHOU L, et al. Biodegradable Metal-Organic-Framework-Gated Organosilica for Tumor-Microenvironment-Unlocked Glutathione-Depletion-Enhanced Synergistic Therapy. Adv Mater. 2022;34(12):2107560. [15] XU N, HU A, PU X, et al. Cu-Chelated Polydopamine Nanoparticles as a Photothermal Medium and “Immunogenic Cell Death” Inducer for combined tumor therapy. J Mater Chem B. 2022;10(16):3104-3118. [16] DONG D, CHENG Z, WANG T, et al. Acid-Degradable Nanocomposite hydrogel and Glucose Oxidase Combination for Killing Bacterial with Photothermal Augmented Chemodynamic Therapy. Int J Biol Macromol. 2023;234:123745. [17] YANG Z, ZHANG L, WEI J, et al. Tumor Acidity-Activatable Photothermal/Fenton Nanoagent for Synergistic Therapy. J Colloid Interface Sci. 2022; 612:355-366. [18] JIA C, GUO Y, WU FG. Chemodynamic Therapy via Fenton and Fenton-Like Nanomaterials: Strategies and Recent Advances. Small. 2022;18(6):2103868. [19] LIU Y, NIU R, DENG R, et al. Multi-enzyme Co-expressed Dual-Atom Nanozymes Induce Cascade Immunogenic Ferroptosis via Activating Interferon-γ and Targeting Arachidonic Acid Metabolism. J Am Chem Soc. 2023;145(16):8965-8978. [20] LIU Z, LIU S, LIU B, et al. Fe(III)-Naphthazarin Metal–Phenolic Networks for Glutathione-Depleting Enhanced Ferroptosis–Apoptosis Combined Cancer Therapy. Small. 2023;19:2207825. [21] MCDONALD PC, CHAFE SC, SUPURAN CT, et al. Cancer Therapeutic Targeting of Hypoxia Induced Carbonic Anhydrase IX: From Bench to Bedside. Cancers. 2022;14(14):3297. [22] GAO D, CHEN T, CHEN S, et al. Targeting Hypoxic Tumors with Hybrid Nanobullets for Oxygen-Independent Synergistic Photothermal and Thermodynamic Therapy. Nanomicro Lett. 2021;13(1):99. [23] QUEEN A, BHUTTO HN, YOUSUF M, et al. Carbonic anhydrase IX: A Tumor Acidification Switch in Heterogeneity and Chemokine Regulation. Semin Cancer Biol. 2022;86:899-913. [24] DI FIORE A, SUPURAN CT, SCALONI A, et al. Post-translational Modifications in Tumor-Associated Carbonic Anhydrases. Amino Acids. 2022;54(4):543-558. [25] CHAFE SC, VIZEACOUMAR FS, VENKATESWARAN G, et al. Genome-Wide Synthetic Lethal Screen Unveils Novel CAIX-NFS1/xCT Axis as a Targetable Vulnerability in Hypoxic Solid Tumors. Sci Adv. 2021;7(35): eabj0364. [26] SARNELLA A, FERRARA Y, AULETTA L, et al. Inhibition of Carbonic Anhydrases IX/XII by SLC-0111 Boosts Cisplatin Effects in Hampering Head and Neck Squamous Carcinoma Cell Growth and Invasion. J Exp Clin Cancer Res. 2022;41(1):122. [27] SU X, WANG WJ, CAO Q, et al. A Carbonic Anhydrase IX (CAIX)-Anchored Rhenium(I) Photosensitizer Evokes Pyroptosis for Enhanced Anti-Tumor Immunity. Angew Chem Int Ed Engl. 2022;61(8):202115800. [28] WANG J, SUN Z, WANG S, et al. Biodegradable Ferrous Sulfide-Based Nanocomposites for Tumor Theranostics through Specific Intratumoral Acidosis-Induced Metabolic Symbiosis Disruption. J Am Chem Soc. 2022;144(43):19884-19895. [29] LIANG S, LIAO G, ZHU W, et al. Manganese-Based Hollow Nanoplatforms for MR Imaging-Guided Cancer Therapies. Biomater Res. 2022;26(1):32. [30] Sanchez-Uriel L, Bonet-Aleta J, Ibarra A, et al. Heterogeneous-Driven Glutathione Oxidation: Defining the Catalytic Role of Chalcopyrite Nanoparticles. J Phys Chem C Nanomater Interfaces. 2023;127(29):14146-14154. [31] AN P, FAN F, GU D, et al. Photothermal-Reinforced and Glutathione-Triggered In Situ Cascaded Nanocatalytic Therapy. J Control Release. 2020;321:734-743. [32] ZHAO J, TIAN Z, ZHAO S, et al. Insights into the Effect of Catalytic Intratumoral Lactate Depletion on Metabolic Reprogramming and Immune Activation for Antitumoral Activity. Adv Sci. 2023;10(4): 2204808. [33] XIE H, LIU X, HUANG Z, et al. Nanoscale Zeolitic Imidazolate Framework (ZIF)-8 in Cancer Theranostics: Current Challenges and Prospects. Cancers (Basel). 2022;14(16):3935. [34] COLUCCIA M, PARISSE V, GUGLIELMI P, et al. Metal-Organic frameworks (MOFs) as Biomolecules Drug Delivery Systems for Anticancer Purposes. Eur J Med Chem. 2022;244:114801. [35] CHEN Y, LYU R, WANG J, et al. Metal-Organic Frameworks Nucleated by Silk Fibroin and Modified with Tumor-Targeting Peptides for Targeted Multimodal Cancer Therapy. Adv Sci. 2023;10(28):2302700. [36] LEI C, LIU XR, CHEN QB, et al. Hyaluronic Acid and Albumin Based Nanoparticles for Drug Delivery. J Control Release. 2022;331:416-433. [37] HASSN MESRATI M, SYAFRUDDIN SE, MOHTAR MA, et al. CD44: A Multifunctional Mediator of Cancer Progression. Biomolecules. 2021; 11(12):1850. [38] ZHANG R, ZHAO X, JIA A, et al. Hyaluronic Acid-Based Prodrug Nanomedicines for Enhanced Tumor Targeting and Therapy: A Review. Int J Biol Macromol. 2023;249:125993. |
| [1] | Yang Yifeng, Ye Nan, Wang Lin, Guo Shuaicheng, Huang Jian. Signaling pathway of dexmedetomidine against ischemia-reperfusion injury [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(9): 1464-1469. |
| [2] | Yue Yun, Wang Peipei, Yuan Zhaohe, He Shengcun, Jia Xusheng, Liu Qian, Li Zhantao, Fu Huiling, Song Fei, Jia Menghui. Effects of croton cream on JNK/p38 MAPK signaling pathway and neuronal apoptosis in cerebral ischemia-reperfusion injury rats [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(8): 1186-1192. |
| [3] | Shen Feiyan, Yao Jixiang, Su Shanshan, Zhao Zhongmin, Tang Weidong. Knockdown of circRNA WD repeat containing protein 1 inhibits proliferation and induces apoptosis of chondrocytes in knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 499-504. |
| [4] | Chen Zepeng, Hou Yonghui, Chen Shudong, Hou Yu, Lin Dingkun. Tauroursodeoxycholic acid treats spinal cord injury by reducing apoptosis of spinal cord neurons under glucose and oxygen deprivation [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(4): 528-534. |
| [5] | Zhang Xinyue, Zhu Liuhui, He Yu, Guan Ying, Zhu Zhouhai, Ren Hui, Yang Xinglong. Neuroprotective mechanism of nicotine in a mouse model of rotenone-induced Parkinson’s disease [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(35): 5612-5617. |
| [6] | Zhou Guanjin, Li Yanan, Li Tao. Neferine inhibits interleukin-1beta-induced chondrocyte apoptosis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(35): 5624-5629. |
| [7] | Zhao Yiting, Zhang Yuxiang, Ma Jie, He Xuejiao. Vascular endothelial growth factor 165/bone morphogenetic protein improves osteoblast injury under hypoxic and reoxygenated conditions [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(35): 5669-5674. |
| [8] | Xu Zheng, Zhao Xiaoqin, Chen Xiaodan, Wang Jiapu, Bao Fenmiao, Yu Liang, Li Junping, Wei Yan. Aerobic exercise upregulates the thioredoxin system and inhibits cardiomyocyte apoptosis in aging rats [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(34): 5508-5515. |
| [9] | Yi Zhi, Zhan Hongwei, Wang Yaobin, Liang Xiaoyuan, Niu Yongkang, Xiang Dejian, Geng Bin, Xia Yayi. Caveolin-1 mediated fluid shear stress regulates proliferation and apoptosis of MC3T3-E1 osteoblasts [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(34): 5440-5445. |
| [10] | Lu Mengya, Wu Xian, She Zeyu, Xia Shuai, Lu Man, Yang Yonghui. Acupotomy prevents knee osteoarthritis in rats by modulating chondrocyte apoptosis in the mitochondrial pathway [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(32): 5190-5195. |
| [11] | Hou Zengtao, Dong Zhiwei, Zhang Jinfeng, Yang Xiaohui, Fan Xiao. Platelet-rich fibrin regulates apoptosis to promote cartilage repair in rats with knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(32): 5167-5171. |
| [12] | Wang Xi, Yu Li, Jia Qiyu, Huang Jinyong, Liu Zebiao, Zhang Jun, Jiayidaer•Dilimulati, Xie Zengru, Ma Hairong. Effects of filament B knockdown on proliferation, migration and apoptosis of mouse MC3T3-E1 cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(32): 5177-5181. |
| [13] | Gao Jie, , , Zou Xingxing, Wen Banghong, Li Yuandi, Su Min, , , Hu Rong, , . Effect of Pax6 gene expression on hydrogen peroxide-induced aging in bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(31): 4921-4925. |
| [14] | Qian Longjie, Su Wenli, Zhu Wenxian, Wang Yixin. SRT1720, an activator of silent information regulator 1, alleviates acute traumatic brain injury in a rat model [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(28): 4447-4454. |
| [15] | Jiang Yu, Xu Lin, Zhao Yalin, Liu Gang, Zhang Yaqi, Bai Huizhong, Ren Jingpei, Zeng Jie, Mu Xiaohong. Effects of Shujin Jiannao Prescription on cell apoptosis in rats with hypoxic-ischemic brain injury [J]. Chinese Journal of Tissue Engineering Research, 2024, 28(28): 4477-4483. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||