Chinese Journal of Tissue Engineering Research ›› 2020, Vol. 24 ›› Issue (13): 2114-2119.doi: 10.3969/j.issn.2095-4344.2052
Previous Articles Next Articles
Biological characteristics and differentiation potential of fibroblasts
Yang Guiran, Wang Fuke, Li Yanlin
- Department of Sports Medicine, First Affiliated Hospital of Kunming Medical University, Kunming 650032, Yunnan Province, China
-
Received:2019-08-08Revised:2019-08-10Accepted:2019-10-24Online:2020-05-08Published:2020-03-11 -
Contact:Wang Fuke, MD, Professor, Department of Sports Medicine, First Affiliated Hospital of Kunming Medical University, Kunming 650032, Yunnan Province, China -
About author:Yang Guiran, Master candidate, Department of Sports Medicine, First Affiliated Hospital of Kunming Medical University, Kunming 650032, Yunnan Province, China -
Supported by:the Yunnan Provincial Science and Technology Department - Kunming Medical University Applied and Basic Research Project (General Program), No. 201701UH00095; the Medical Academic Leader Training Program of Yunnan Province, No. D2016-39
CLC Number:
Cite this article
Yang Guiran, Wang Fuke, Li Yanlin . Biological characteristics and differentiation potential of fibroblasts[J]. Chinese Journal of Tissue Engineering Research, 2020, 24(13): 2114-2119.
share this article
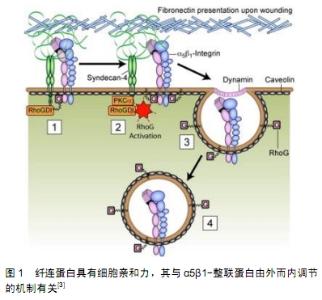
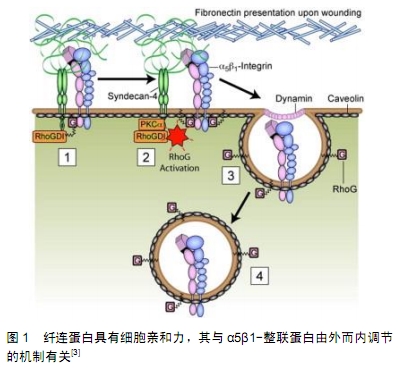
2.1 成纤维细胞的来源和特征 成纤维细胞也称为纤维母细胞,是疏松结缔组织中最常见的细胞,但通常致密结缔组织中成纤维细胞的数量要多于同等体积疏松结缔组织中成纤维细胞的数量,故分离培养成纤维细胞主要以真皮等致密结缔组织为取材部位[2]。成纤维细胞在机体内分布广、数量多,其细胞体积大且形态多样多变,以梭形、鹿角形或星形为主,还能随不同的条件随时改变,细胞核比较大,为椭圆形,核仁明显;胞质嗜弱碱性,含有丰富而发达的粗面内质网、高尔基复合体以及游离核糖体,所以它具有强大的合成分泌蛋白质的功能。由于成纤维细胞起源于胚胎中胚层的间充质,故细胞增殖能力强且代谢旺盛,可体外培养、多次传代,还具备合成胶原纤维蛋白、粘连蛋白以及分泌胶原纤维、弹性纤维、网状纤维的能力。当成纤维细胞成熟时或者处于静止状态,其胞体体积减小,呈长梭形,粗面内质网和高尔基复合体减少,被称为纤维细胞。在外伤等因素刺激下,部分纤维细胞重新转回幼稚的成纤维细胞,其分泌及合成功能活性随之恢复,从而参与组织损伤后的修复[3],见图1。在结缔组织中,有少量具有分化潜能的间充质细胞也能在创伤修复等情况下增殖分化为成纤维细胞[4]。成纤维细胞只是从形态上被定义的一类细胞,目前仍然缺乏公认的标准来定义成纤维细胞,还有学者直接将成纤维细胞列入干细胞行列[5]。 "

2.2 成纤维细胞的多向分化性能 2.2.1 成纤维细胞诱导分化为诱导性多能干细胞(induced pluripotent stem cells,iPSCs) 2006年,由日本Takahashi团队将4种转录因子(Oct3/4、Sox2、c-Myc和Klf4)导入小鼠成纤维细胞,通过重编程获得了诱导性多能干细胞——一种和胚胎干细胞相媲美的全能干细胞[6]。随后,美国和日本2个研究组又分别采用不同的转录因子将人皮肤成纤维细胞转化为诱导性多能干细胞,可向3个胚层的所有细胞分化[7-8],包括成骨细胞、成软骨细胞、脂肪细胞、肌和肌腱细胞、内皮细胞、许旺细胞[9]、肝细胞、神经细胞、心肌细胞等多种细 胞[10]。 2.2.2 成纤维细胞分化为软骨细胞或成骨细胞 成纤维细胞虽与成骨细胞来源相同,却具有成骨细胞不具备的增殖特性,在一定的诱导条件下成纤维细胞还可向成骨细胞和破骨细胞分化,表达成骨标志物。例如,牙周韧带成纤维细胞能在不同类型生物力学刺激下作出胞内信号的应答,对其自身向成骨方向分化起到调控作 用[11]。研究发现,在人牙周膜成纤维细胞和人微血管内皮细胞体外共培养模型中有早期成骨分化基因Twist-1、Twist-2、tafazzin-5和msh homebox 2等的表达,为该共培养体系中成骨细胞分化的发生提供了证据[12]。重组骨形态发生蛋白9可通过碱性磷酸酶活性、Runt相关转录因子2/核心结合因子1表达增加从而诱导人牙周韧带成纤维细胞的成骨分化[13]。有明确报道,骨形态发生蛋白7可通过Smad和MAPK通路增强人真皮成纤维细胞的成骨分化作用[14],小鼠皮肤成纤维细胞在c-Myc、Klf4及SOX9基因的诱导下可高效地向软骨分化[15]。此外,已有报道指出人成纤维细胞在RNUX2、OSX、OCN、BSP和Col1a基因的诱导下,能表达成骨标志物,并可诱导为功能完善的成骨细胞,目前已利用确定的转录因子如Osterix、Runx2、Oct4、L-myc成功将成纤维细胞直接转化为成骨细胞[16]。SOMMAR等[17]在体外通过组织工程技术构建的仿生3D培养模型中,皮肤成纤维细胞均可向成骨及成软骨分化。因此,成纤维细胞具有应用于骨缺损及其相关疾病治疗的巨大潜力。 2.2.3 成纤维细胞分化为肌细胞 有学者研究发现,纳米银能诱导成纤维细胞向肌纤维母细胞分化,以促进皮肤伤口肉芽组织的收缩、加速伤口的愈合[18];凝集素也能诱导成纤维细胞向肌纤维细胞分化同时分泌细胞外基质促进伤口愈合[19],而且当提供一定生物力学刺激的情况下,成肌腱纤维如成熟肌腱组织一样,排列更为整齐紧密以抵抗外部机械拉力[20]。经修饰的肌纤维细胞分化蛋白mRNA转染人成纤维细胞,也是一种高效直接的诱导方法,最终使成纤维细胞向肌细胞方向分化[21]。此外,在肌腱水化胶细胞培养基条件下对比成纤维细胞和脂肪干细胞,发现成纤维细胞生存时间更长,适用于修复慢性肌腱损伤[22]。 2.2.4 成纤维细胞分化为脂肪细胞 起源于中胚层间质的成纤维细胞与脂肪干细胞表达一些相同的标志物CD29、CD44、CD71d、CD73等,且在一定诱导条件下能分化为脂肪组织,例如有研究发现具有骨相关毒性的药物罗格列酮可诱导小鼠胚胎成纤维细胞成脂分 化[23],并且成纤维细胞生长因子亦能加强脂肪干细胞的脂向分化性能[24],可见成纤维细胞对皮下脂肪组织也有促进生长的作用,但也从一定程度上参与了一些疾病发生发展的病理转化。 2.2.5 成纤维细胞分化为神经干细胞及神经元 研究表明,由于NEUROD2基因促进表达的miR-9/9*和miR-124(miR-9/9*-124)产生诱导作用,人成纤维细胞在有神经干细胞分化生长相关基因转染条件下,可直接诱导分化为成熟的神经元[25]。另外还有学者发现,DNA双氧酶Tet3可通过介导DNA去甲基化,快速地诱导成纤维细胞成为功能神经元,表达成熟的神经元标志物[26]。相关研究结论也证明成纤维细胞因子具有较强的促进神经干细胞增殖和分化的作用,是重要的神经促生长因子,其促进细胞增殖和分化的作用呈浓度依赖型。但与其他类型干细胞比较,成纤维细胞向神经细胞的分化有着途径复杂、诱导率低的特点,仍需进一步深入探究。 2.3 共培养中成纤维细胞对干细胞增殖分化的影响及作用 2.3.1 成纤维细胞与胚胎干细胞共培养 有学者发现,牙周韧带成纤维细胞通过分泌成纤维细胞因子参与MAPKs介导的信号通路调控胚胎干细胞的增殖和成骨分化[27],除了成纤维细胞因子4、成纤维细胞因子7以外,牙周韧带成纤维细胞亦能产生转化生长因子、骨形态发生蛋白和一些其他细胞因子共同形成微环境从而加强其调控作用。还有研究证实,牙周韧带成纤维细胞诱导胚胎干细胞的增殖和分化是通过JNK和/或ERK信号通路(MAPKs的亚分类)激活介导的[28]。因此,牙周韧带成纤维细胞对胚胎干细胞的增殖分化有重要的调控作用。 2.3.2 成纤维细胞与牙髓干细胞共培养 研究发现,在无外源动物血清的条件下加入血管内皮生长因子和碱性成纤维细胞生长因子,小鼠成纤维细胞来源的诱导性多能干细胞可向血管内皮细胞定向分化,将分化后的细胞与牙髓干细胞共培养,内皮样细胞有形成血管的倾 向[29]。另外,有研究人员成功构建了可注射的纳米纤维微球[30],其具有良好的组织相容性,在与牙髓干细胞共培养时可提供良好的基质微环境,其中起到主要作用的是纤维微球分泌的成纤维细胞因子2,有效促进其成牙向分化,为最终达到牙本质修复目的奠定细胞基础。 2.3.3 成纤维细胞与间充质干细胞共培养 在共培养成纤维细胞以及由转化生长因子β1、血小板衍生生长因子BB和骨形态发生蛋白4组成的生长因子混合物的条件下,间充质干细胞可自主向血管平滑肌细胞分化,其中标记基因的转录增加,收缩装置蛋白的表达上调,并且血管平滑肌细胞表型的功能活性增强[31]。这项研究验证了成纤维细胞及细胞外基质提供的微环境具有直接将间充质干细胞诱导分化为成熟的、具有功能的血管平滑肌表型的能力,类似于可溶性生长因子的性能;而间充质干细胞本身就具有多项分化潜能,故在共培养体系中微环境的诱导及调控就显得极为重要。 (1)成纤维细胞对骨髓间充质干细胞的影响:研究发现,通过特定成纤维细胞生长因子预处理的骨髓间充质干细胞产生细胞外钙结节的数量、细胞内脂滴的数量、细胞酸性黏多糖的表达明显增加,且有成纤维细胞生长因子的微环境可以维持多次传代骨髓间充质干细胞的干细胞特性[32]。还有类似相关研究表明,碱性成纤维细胞生长因子转染的骨髓间充质干细胞与韧带成纤维细胞通过3D共培养,一方面促进了韧带成纤维细胞增殖的同时抑制了其胶原合成能力,另一方面促进骨髓间充质干细胞增殖的同时增强了其向韧带成纤维细胞分化的能力[33]。还有研究证明,加载有碱性成纤维细胞生长因子的壳聚糖能够将成年大鼠骨髓间充质干细胞高比例诱导分化为神经元样细胞[34],为骨髓间充质干细胞自体移植治疗神经退行性疾病和中枢神经系统疾病提供了一定的理论和实验依据。 (2)成纤维细胞对脂肪间充质干细胞的影响:在与正常人类成纤维细胞共培养条件下,脂肪间充质干细胞的MMP1、MMP2基因表达升高,TIMP1、TIMP2蛋白表达升高,故当受外部辐射时细胞增殖受损程度与对照组相比大幅减少[35]。还有研究提示,碱性成纤维细胞生长因子可促进透明质酸钠支架中的人脂肪干细胞向血管内皮迁移、分化,这意味着在人脂肪干细胞-透明质酸钠复合物中加入碱性成纤维细胞生长因子可加速移植物血运重建而减缓移植物的吸收,更好地构建组织工程脂肪,从而达到较为理想的修复重建效果[36]。 2.3.4 成纤维细胞对神经干细胞的影响 成纤维细胞分泌的碱性成纤维细胞生长因子具有强大的促神经干细胞增殖作用,又能增强中枢神经系统不同区域的神经元前体细胞的再生能力,并且具有浓度依赖性[37],而反向来说,碱性成纤维细胞生长因子亦有助于诱导表皮干细胞向神经细胞分化,而且该诱导作用具有一定的细胞密度依赖性。故在不等同的微环境条件下,细胞间相互作用不尽相同,甚至完全相反。成纤维细胞对于神经干细胞的作用机制复杂且灵活,具有多样性。 "

| [1] DASTAGIR K, REIMERS K, LAZARIDIS A, et al. Murine embryonic fibroblast cell lines differentiate into three mesenchymal lineages to different extents: new models to investigate differentiation processes. Cell Reprogram. 2014;16(4):241-252. [2] ALT E, YAN Y, GEHMERT S, et al. Fibroblasts share mesenchymal phenotypes with stem cells, but lack their differentiation and colony-forming potential. Biol Cell. 2011; 103(4):197-208. [3] BASS MD, WILLIAMSON RC, NUNAN RD, et al. A syndecan-4 hair trigger initiates wound healing through caveolin- and RhoG-regulated integrin endocytosis. Dev Cell. 2011;21(4):681-693. [4] ZHOU ZQ, CHEN Y, CHAI M, et al. Adipose extracellular matrix promotes skin wound healing by inducing the differentiation of adipose‑derived stem cells into fibroblasts. Int J Mol Med. 2019;43(2):890-900. [5] TRUFFI M, MAZZUCCHELLI S, BONIZZI A, et al. Nano-Strategies to Target Breast Cancer-Associated Fibroblasts: Rearranging the Tumor Microenvironment to Achieve Antitumor Efficacy. Int J Mol Sci. 2019;20(6): E1263. [6] TAKAHASHI K, YAMANAKA S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663-676. [7] TAKAHASHI K, TANABE K, OHNUKI M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861-872. [8] YU J, VODYANIK MA, SMUGA-OTTO K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917-1920. [9] SOWA Y, KISHIDA T, TOMITA K, et al. Direct Conversion of Human Fibroblasts into Schwann Cells that Facilitate Regeneration of Injured Peripheral Nerve In Vivo. Stem Cells Transl Med. 2017;6(4):1207-1216. [10] 杨晓双,胡鹏,王达利,等.兔自体真皮成纤维细胞对增生性瘢痕作用的实验研究[J].中华整形外科杂志, 2018, 34(9):758-768. [11] LI M, ZHANG C, YANG Y. Effects of mechanical forces on osteogenesis and osteoclastogenesis in human periodontal ligament fibroblasts: A systematic review of in vitro studies. Bone Joint Res. 2019;8(1):19-31. [12] NEELEY WW, CARNES DL, COCHRAN DL. Osteogenesis in an in vitro coculture of human periodontal ligament fibroblasts and human microvascular endothelial cells. J Periodontol. 2010;81(1):139-149. [13] FUCHIGAMI S, NAKAMURA T, FURUE K, et al. Recombinant human bone morphogenetic protein-9 potently induces osteogenic differentiation of human periodontal ligament fibroblasts. Eur J Oral Sci. 2016;124(2):151-157. [14] CHEN F, BI D, CHENG C, et al. Bone morphogenetic protein 7 enhances the osteogenic differentiation of human dermal-derived CD105+ fibroblast cells through the Smad and MAPK pathways. Int J Mol Med. 2019;43(1):37-46. [15] BOUGIOUKLI S, SUGIYAMA O, PANNELL W, et al. Gene Therapy for Bone Repair Using Human Cells: Superior Osteogenic Potential of Bone Morphogenetic Protein 2-Transduced Mesenchymal Stem Cells Derived from Adipose Tissue Compared to Bone Marrow. Hum Gene Ther. 2018;29(4):507-519. [16] CHANG Y, CHO B, KIM S, et al. Direct conversion of fibroblasts to osteoblasts as a novel strategy for bone regeneration in elderly individuals. Exp Mol Med. 2019;51(5):54. [17] SOMMAR P, PETTERSSON S, NESS C, et al. Engineering three-dimensional cartilage- and bone-like tissues using human dermal fibroblasts and macroporous gelatine microcarriers. J Plast Reconstr Aesthet Surg. 2010;63(6): 1036-1046. [18] 杜娟,刘雪来.皮肤成纤维细胞“干”性特征及其在创伤治疗中的应用前景[J].中华损伤与修复杂志(电子版),2018,13(3):230-233. [19] CHO N, RAZIPOUR SE, MCCAIN ML. Featured Article: TGF-β1 dominates extracellular matrix rigidity for inducing differentiation of human cardiac fibroblasts to myofibroblasts. Exp Biol Med (Maywood). 2018;243(7):601-612. [20] DENG D, LIU W, XU F, et al. Engineering human neo-tendon tissue in vitro with human dermal fibroblasts under static mechanical strain. Biomaterials. 2009;30(35):6724-6730. [21] PRESKEY D, ALLISON TF, JONES M, et al. Synthetically modified mRNA for efficient and fast human iPS cell generation and direct transdifferentiation to myoblasts. Biochem Biophys Res Commun. 2016;473(3):743-751. [22] CHATTOPADHYAY A, GALVEZ MG, BACHMANN M, et al. Tendon Regeneration with Tendon Hydrogel-Based Cell Delivery: A Comparison of Fibroblasts and Adipose-Derived Stem Cells. Plast Reconstr Surg. 2016;138(3):617-626. [23] SHAO Y, CHEN QZ, ZENG YH, et al. All-trans retinoic acid shifts rosiglitazone-induced adipogenic differentiation to osteogenic differentiation in mouse embryonic fibroblasts. Int J Mol Med. 2016;38(6):1693-1702. [24] BROHEM CA, DE CARVALHO CM, RADOSKI CL, et al. Comparison between fibroblasts and mesenchymal stem cells derived from dermal and adipose tissue. Int J Cosmet Sci. 2013;35(5):448-457. [25] YOO AS, SUN AX, LI L, et al. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature. 2011;476(7359): 228-231. [26] WU W, JIN YQ, GAO Z. Directly reprogramming fibroblasts into adipogenic, neurogenic and hepatogenic differentiation lineages by defined factors. Exp Ther Med. 2017;13(6): 2685-2690. [27] LI M, ZHANG C, YANG Y. Effects of mechanical forces on osteogenesis and osteoclastogenesis in human periodontal ligament fibroblasts: A systematic review of in vitro studies. Bone Joint Res. 2019;8(1):19-31. [28] KOOK SH, JEON YM, PARK SS, et al. Periodontal fibroblasts modulate proliferation and osteogenic differentiation of embryonic stem cells through production of fibroblast growth factors. J Periodontol. 2014;85(4):645-654. [29] ONO M, MASAKI A, MAEDA A, et al. CCN4/WISP1 controls cutaneous wound healing by modulating proliferation, migration and ECM expression in dermal fibroblasts via α5β1 and TNFα. Matrix Biol. 2018;68-69:533-546. [30] NEELEY WW, CARNES DL, COCHRAN DL. Osteogenesis in an in vitro coculture of human periodontal ligament fibroblasts and human microvascular endothelial cells. J Periodontol. 2010;81(1):139-149. [31] LI N, SANYOUR H, REMUND T, et al. Vascular extracellular matrix and fibroblasts-coculture directed differentiation of human mesenchymal stem cells toward smooth muscle-like cells for vascular tissue engineering. Mater Sci Eng C Mater Biol Appl. 2018;93:61-69. [32] 宋明宇,杨勇,吴华,等.成纤维细胞生长因子处理多次传代培养骨髓间充质干细胞的增殖与分化[J].中国组织工程研究, 2017, 21(25):3937-3942。 [33] 李斌,陈廖斌,齐勇建,等.碱性成纤维细胞生长因子基因转染骨髓干细胞与韧带成纤维细胞共培养效应[J].中国组织工程研究, 2015,19(28):4429-4434. [34] GU W, ZHANG F, XUE Q, et al. Bone mesenchymal stromal cells stimulate neurite outgrowth of spinal neurons by secreting neurotrophic factors. Neurol Res. 2012;34(2): 172-180. [35] HAUBNER F, MUSCHTER D, POHL F, et al. A Co-Culture Model of Fibroblasts and Adipose Tissue-Derived Stem Cells Reveals New Insights into Impaired Wound Healing After Radiotherapy. Int J Mol Sci. 2015;16(11):25947-25958. [36] ZHOU ZQ, CHEN Y, CHAI M, et al. Adipose extracellular matrix promotes skin wound healing by inducing the differentiation of adipose‑derived stem cells into fibroblasts. Int J Mol Med. 2019;43(2):890-900. [37] 许正伟,贺宝荣,刘团江,等.碱性成纤维细胞因子对SD大鼠表皮干细胞分化为神经干细胞的影响[J].安徽医药, 2019, 23(3) : 445-449. [38] WANG Q, DING G, XU X. Immunomodulatory functions of mesenchymal stem cells and possible mechanisms. Histol Histopathol. 2016;31(9):949-959. [39] HUANG HI, CHEN SK, LING QD, et al. Multilineage differentiation potential of fibroblast-like stromal cells derived from human skin. Tissue Eng Part A. 2010;16(5):1491-1501. [40] HIROBE T, SHIBATA T, SATO K. Human fibroblasts treated with hydrogen peroxide stimulate human melanoblast proliferation and melanocyte differentiation, but inhibit melanocyte proliferation in serum-free co-culture system. J Dermatol Sci. 2016;84(3):282-295. [41] DENU RA, NEMCEK S, BLOOM DD, et al. Fibroblasts and Mesenchymal Stromal/Stem Cells Are Phenotypically Indistinguishable. Acta Haematol. 2016;136(2):85-97. [42] ALT E, YAN Y, GEHMERT S, et al. Fibroblasts share mesenchymal phenotypes with stem cells, but lack their differentiation and colony-forming potential. Biol Cell. 2011; 103(4):197-208. [43] 陈伟,戴茹,华薇,等.皮肤成纤维细胞是一种间充质干细胞吗[J].中国皮肤性病学杂志, 2018, 32(10):1208-1212. [44] BI D, CHEN FG, ZHANG WJ, et al. Differentiation of human multipotent dermal fibroblasts into islet-like cell clusters. BMC Cell Biol. 2010;11:46. [45] ZHANG Z, CHAI W, XIONG R, et al. Printing-induced cell injury evaluation during laser printing of 3T3 mouse fibroblasts. Biofabrication. 2017;9(2):025038. [46] CHUI CY, MOUTHUY PA, YE H. Direct electrospinning of poly(vinyl butyral) onto human dermal fibroblasts using a portable device. Biotechnol Lett. 2018;40(4):737-744. [47] XU R, HU X, YU X, et al. Micro-/nano-topography of selective laser melting titanium enhances adhesion and proliferation and regulates adhesion-related gene expressions of human gingival fibroblasts and human gingival epithelial cells. Int J Nanomedicine. 2018;13:5045-5057. [48] TRUFFI M, MAZZUCCHELLI S, BONIZZI A, et al. Nano-Strategies to Target Breast Cancer-Associated Fibroblasts: Rearranging the Tumor Microenvironment to Achieve Antitumor Efficacy. Int J Mol Sci. 2019;20(6): E1263. |
| [1] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [2] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [3] | Zhang Xiumei, Zhai Yunkai, Zhao Jie, Zhao Meng. Research hotspots of organoid models in recent 10 years: a search in domestic and foreign databases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1249-1255. |
| [4] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [5] | Shi Yangyang, Qin Yingfei, Wu Fuling, He Xiao, Zhang Xuejing. Pretreatment of placental mesenchymal stem cells to prevent bronchiolitis in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 991-995. |
| [6] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [7] | Fan Quanbao, Luo Huina, Wang Bingyun, Chen Shengfeng, Cui Lianxu, Jiang Wenkang, Zhao Mingming, Wang Jingjing, Luo Dongzhang, Chen Zhisheng, Bai Yinshan, Liu Canying, Zhang Hui. Biological characteristics of canine adipose-derived mesenchymal stem cells cultured in hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1002-1007. |
| [8] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [9] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [10] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| [11] | Duan Liyun, Cao Xiaocang. Human placenta mesenchymal stem cells-derived extracellular vesicles regulate collagen deposition in intestinal mucosa of mice with colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1026-1031. |
| [12] | Pei Lili, Sun Guicai, Wang Di. Salvianolic acid B inhibits oxidative damage of bone marrow mesenchymal stem cells and promotes differentiation into cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1032-1036. |
| [13] | Guan Qian, Luan Zuo, Ye Dou, Yang Yinxiang, Wang Zhaoyan, Wang Qian, Yao Ruiqin. Morphological changes in human oligodendrocyte progenitor cells during passage [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1045-1049. |
| [14] | Wang Zhengdong, Huang Na, Chen Jingxian, Zheng Zuobing, Hu Xinyu, Li Mei, Su Xiao, Su Xuesen, Yan Nan. Inhibitory effects of sodium butyrate on microglial activation and expression of inflammatory factors induced by fluorosis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1075-1080. |
| [15] | Wang Xianyao, Guan Yalin, Liu Zhongshan. Strategies for improving the therapeutic efficacy of mesenchymal stem cells in the treatment of nonhealing wounds [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1081-1087. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||