Chinese Journal of Tissue Engineering Research ›› 2019, Vol. 23 ›› Issue (2): 304-309.doi: 10.3969/j.issn.2095-4344.0656
Previous Articles Next Articles
Application of graphene in bone tissue engineering
Zhang Jun, You Qi, Xiong Huazhang, Zou Gang, Jin Ying, Ge Zhen, Liu Yi
- First Department of Orthopedics, Affiliated Hospital of Zunyi Medical University, Zunyi Medical University (College), Zunyi 563000, Guizhou Province, China
-
Received:2018-07-26Online:2019-01-18Published:2019-01-18 -
Contact:Liu Yi, Professor, Master’s supervisor, First Department of Orthopedics, Affiliated Hospital of Zunyi Medical University, Zunyi Medical University (College), Zunyi 563000, Guizhou Province, China -
About author:Zhang Jun, Master candidate, First Department of Orthopedics, Affiliated Hospital of Zunyi Medical University, Zunyi Medical University (College), Zunyi 563000, Guizhou Province, China -
Supported by:the Science & Technology Program of Guizhou Province, No. LH[2016]7477 (to XHZ), LH[2017]7015 (to ZG); the Project of the Affiliated Hospital of Zunyi Medical University, No. (2016)34 (to JY)
CLC Number:
Cite this article
Zhang Jun, You Qi, Xiong Huazhang, Zou Gang, Jin Ying, Ge Zhen, Liu Yi . Application of graphene in bone tissue engineering[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(2): 304-309.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks

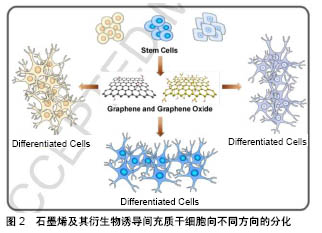
2.1 石墨烯的一般特性 石墨烯是由碳原子以sp2杂化轨道而形成的单层二维平面晶体,其外观呈六角蜂巢体状[1]。石墨烯最早是由英国物理学家Andre和Konstantin分离发现的[2],其厚度只有0.335 nm,是目前世界上最薄的新型纳米材料[3]。由于其晶体结构比较稳定,所以石墨烯的稳定性、导电性、导热性、机械强度均较普通二维材料高[4],在生物医学、组织工程学应用较广泛[5-8]。最近几年来发现,经过功能修饰后的石墨烯具有更加优良的生物相容性和亲水性,使其在生物工程中的应用得到了进一步推广。此外,石墨烯还具有刺激细胞增殖分化的能力,因此在组织工程中特别是组织工程骨、促进骨再生方面具有良好的前景[5]。 2.2 石墨烯的生物相容性 生物相容性是评价一种材料的基础,也是决定该材料能否应用的先决条件。当前检测一种材料生物相容性的方法较多,目前使用比较多的是体外细胞培养方法来检测材料对细胞增殖活性的影响,即该材料是否具有细胞毒性。该方法操作简便,并且敏感性较高[9]。 许多研究发现,低浓度石墨烯对细胞的增殖和细胞活性没有较大影响,但如果浓度过高则会影响细胞的生物活性。Zhang等[10]观察不同质量浓度(0-100 mg/L)石墨烯悬液和单壁碳纳米管悬液对PC12细胞增殖代谢的影响,结果显示:当石墨烯质量浓度< 0.01 mg/L时对细胞几乎不产生毒性,PC12细胞的活性没有受到影响;但当石墨烯质量浓度达到100 mg/L时,PC12细胞产生活性氧的水平和活性(细胞凋亡标志物)明显增高,释放乳酸脱氢酶也开始增多,提示石墨烯对细胞活性的影响与其浓度有关。并且进一步的研究推断:高质量浓度石墨烯实现细胞毒性作用,主要是通过caspase 3介导的细胞凋亡机制和氧化应激反应来实现的。Kalbacova等[11]发现,石墨烯不仅能够促进间充质基质细胞和成骨细胞的生长,还能够诱导间充质基质细胞增殖分化为成骨细胞,提示石墨烯能够诱导间充质干细胞的成骨分化,在骨再生领域具有良好的应用前景。 石墨烯的生物相容性除了与其本身性质相关,还与其复合物有关。不同材料与石墨烯复合时,其生物相容性也会发生改变。徐宏杨等[12]将氧化石墨烯与双端氨基聚乙二醇4000相结合,观察其对小鼠成纤维细胞L929生存率的影响,结果显示:在经聚乙二醇4000修饰氧化石墨烯上孵育的L929细胞生存率高于未经修饰的氧化石墨烯,提示经过聚乙二醇4000修饰可降低氧化石墨烯的细胞毒性,其作用机制可能与聚乙二醇4000化可拮抗细胞内氧化应激损伤有关。同时Liao等[13]发现石墨烯对细胞生物活性的影响还与其所在的状态、颗粒大小、表面带电性有关。 2.3 石墨烯的可降解性 任何一种生物材料,尤其是医用植入物的安全性能和临床效果的一个关键指标就是植入物的可降解性,以及降解产物是否能被安全地吸收或代谢。石墨烯作为一种广泛应用的生物材料,其在生物体内的降解研究起来比较困难,主要原因是生物体具有非常复杂的脉管系统,并且降解过程中石墨烯的瞬时化学结构改变很难检测到[14-15]。Girish等[16]通过静脉注射石墨烯的方法,利用拉曼分析描绘了石墨烯降解过程中在小鼠肝、肾、肺等器官的分布,结果显示从第8天开始可观察到石墨烯从周边向器官中心扩散,一直持续到3个月。石墨烯的降解主要是依靠巨噬细胞的吞噬作用,此外,免疫反应的基因表达分析也显示了巨噬细胞的免疫应答作用。Schinwald等[17]使小鼠吸入未氧化的石墨烯晶体,6周后观察其肺部免疫情况,同样采用拉曼光谱观察石墨烯在小鼠肺部的滞留情况,结果发现在中期检测时没有免疫效应,同时也没有石墨烯的滞留,说明石墨烯在生物体内有自我降解的可能,为其在生物医学的研究和应用提供了理论基础。 2.4 石墨烯在骨组织工程中的应用 2.4.1 石墨烯对间充质干细胞分化成骨的影响 已发现石墨烯不仅影响细胞的附着、迁移和增殖,而且还能促进干细胞向不同谱系的分化[18-20]。很多研究已证实了石墨烯的骨生物活性,例如:即使不使用成骨培养基,在石墨烯上培养的间充质干细胞也可展现出更高水平的矿化,并且上调成骨相关基因和蛋白质表达,包括RUNX2、胶原蛋白和骨钙素等;在成骨诱导剂存在下,在石墨烯上培养的间充质干细胞矿化比对照上更加明显[21]。一方面,由于石墨烯对间充质干细胞或成骨细胞的毒性较小,甚至没有毒性,因此可提高这些细胞在石墨烯材料上的吸附和增殖[22];另一方面,石墨烯具有较强的非共价结合能力,使之作为成骨诱导剂的富集平台,进而能够诱导骨髓间充质干细胞的成骨分化[23]。Elkhenany等[24]将山羊骨髓间质干细胞放置于不加任何生长因子和激素的石墨烯培养基中,惊奇的发现石墨烯不仅能够能促进山羊骨髓间质干细胞的增殖,还能诱导其成骨分化。Lee等和Nayak等[25-26]将人间充质干细胞接种在加有成骨诱导液的石墨烯薄膜上,观察石墨烯薄膜对间充质干细胞的影响,结果发现:石墨烯不仅不会影响间充质干细胞的生长,而且还能够浓集诱导液的有效成骨成分,具有与成骨生长因子同样的作用,加速间充质干细胞的成骨分化,诱导成骨和矿化,提出了石墨烯作为骨组织工程支架材料的可行性。吕成奇等[27]自制了石墨烯水凝胶,然后将人脂肪干细胞接种在自制石墨烯水凝胶上,并且不加成骨诱导液培养,来观察该材料诱导成骨的能力。实验结果显示在不加成骨诱导液的情况下,石墨烯水凝胶可在成骨分化的早、中、晚各个时期对人脂肪干细胞均有诱导作用。Liu等[28]的研究揭示了单层石墨烯可在体外和体内促进人间充质干细胞的成骨分化,主要是通过通过抑制RBP2的表达,上调骨生成相关基因启动子区域H3K4的甲基化水平,促进体外和体内人间充质干细胞的成骨分化。石墨烯及其衍生物诱导间充质干细胞向不同方向的分化,见图2[29]。"
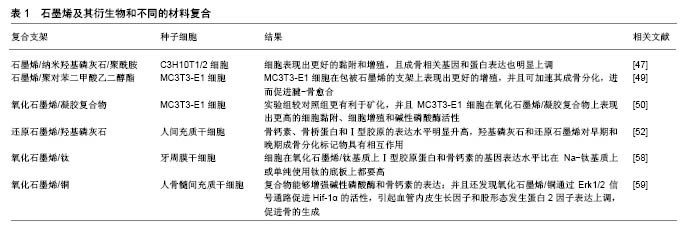
2.4.2 石墨烯及其衍生物的化学修饰和改性 尽管石墨烯可单独诱导成骨和促进骨的形成,但它也可进行化学修饰或与其他材料结合,以进一步增强骨的生物活 性[30],例如:与其他聚合物[31-36]、矿物质[37-42]、金属结合[43-47]。 石墨烯和聚合物复合:Zhang等[48]将鼠骨髓间充质干细胞系C3H10T1/2细胞接种于石墨烯修饰的纳米羟基磷灰石/聚酰胺复合材料上,发现细胞表现出更好的黏附和增殖,并且成骨相关基因和蛋白表达也明显上调。其原因可能是石墨烯增强了生长因子和含苯环化学物质的吸附,如:β-甘油磷酸酯和地塞米松。地塞米松是一种合成的糖皮质激素,可改变成骨过程中所需的许多蛋白质和酶的表达水平,石墨烯较强吸附塞米松的能力,可能归因于地塞米松芳香族结构与石墨烯结构之间的π-π键。Nayak等[49]将石墨烯分别涂在聚二甲基硅氧烷、聚对苯二甲酸乙二醇酯、载玻片和硅晶片上,然后比较了骨形态发生蛋白2和石墨烯对骨生成的影响,结果提示在没有骨形态发生蛋白2的情况下,石墨烯涂层显著增加了所有底板上人间充质干细胞的成骨分化,而这种增强作用对于较硬底板(硅晶片和载玻片)更为显着;另一方面,在骨形态发生蛋白2存在的情况下,观察到在较坚硬材料上涂覆石墨烯不会进一步增加钙沉积物的产生,但在较软材料上(聚二甲基硅氧烷、聚对苯二甲酸乙二醇酯)涂覆石墨烯则明显增加钙的沉积和矿化,这表明石墨烯本身就能促进人间充质干细胞的成骨分化,而不需要添加其他成骨诱导因子。 由聚对苯二甲酸乙二醇酯制成的人工韧带由于缺乏良好的生物相容性,因此其在临床的应用受到了很大限制。Wang等[50]将石墨烯涂在聚对苯二甲酸乙二醇酯表面,来观察是否可改变其生物相容性并加速移植物的腱-骨愈合,体外实验发现,MC3T3-E1细胞在包被石墨烯的支架上表现出更好的增殖,并且可加速其成骨分化,进而促进腱-骨愈合。进一步的体内实验结果显示,石墨烯包覆的聚对苯二甲酸乙二醇酯人工韧带微观结构参数、骨平均矿物沉积速率和生物力学性能显著高于对照组,间接提示石墨烯具有促进骨生成的作用。Liu等[51]通过明胶修饰氧化石墨烯来观察在骨形成过程中细胞外基质的变化,发现氧化石墨烯/凝胶复合物较对照组更有利于矿化,并且MC3T3-E1细胞在氧化石墨烯/凝胶复合物上表现出更高的细胞黏附、细胞增殖和碱性磷酸酶活性。通过扫描电镜和茜素红染色进一步证实了氧化石墨烯/凝胶复合物有促进天然类骨质基质最终沉积的观点,提示氧化石墨烯/凝胶复合物能够促进骨的形成,可作为骨缺损修复的支架,在骨科领域将会有更好的应用前景。 石墨烯和矿物质复合:研究发现石墨烯和一些矿物质复合后也能进一步改善干细胞的成骨能力。一些研究者通过将羟基磷灰石与还原石墨烯混合在一起使用,发现骨钙素、骨桥蛋白和Ⅰ型胶原的表达水平明显升高,并且还发现羟基磷灰石和还原石墨烯对早期和晚期成骨分化标记物具有相互作用[51-54]。因此,暴露于还原石墨烯-羟基磷灰石的细胞表现出较高的成骨效应。但也有研究发现,尽管使用20%羟基磷灰石/还原石墨烯复合支架能够上调Runx2的相对表达,其值在第7天时升高达到最大值,但在第14天开始下降[52]。这种现象可能是由于Runx2作为一种关键的转录因子在骨形成的早期发挥作用[53]。Oyefusi等[55]将羟基磷灰石吸附在石墨烯表面,并在不同温度(34,39 ℃)下处理不同质量浓度(200,400 μg/L)的细胞,发现在39 ℃条件下,用石墨烯/羟基磷灰石复合物处理质量浓度为400 μg/L的细胞,比在34 ℃时表现出更好的成骨效应,骨钙素的表达更加显著。Tatavarty等[56]将间充质干细胞分别接种到氧化石墨烯/磷酸钙复合物、石墨烯、磷酸钙上,发现间充质干细胞在氧化石墨烯/磷酸钙复合物上表现出较高水平的碱性磷酸酶活性和骨钙素表达,这表明氧化石墨烯和磷酸钙能够协同促进骨的生成[57]。 石墨烯和金属复合:将石墨烯掺入金属来促进干细胞分化的研究也取得了不错的结果,并且已有研究证实石墨烯有助于金属的持续释放[58]。许多研究使用钛与石墨烯结合来增强骨的形成[59-63]。已证明钛的表面粗糙度和亲水性可影响Wnt通路和信号分子,促进成骨分化[64]。Zhou等[59]和La等[60]的研究表明,细胞在氧化石氧化石墨烯/钛基质上,Ⅰ型胶原蛋白和骨钙素的基因表达水平比在Na-钛基质上或单纯钛底板上都要高。此外,将Fn结合到氧化石墨烯/钛复合物上,可产生比氧化石墨烯/钛复合物更高的骨形成水平,该结果也已通过碱性磷酸酶活性和ARS染色证实。同样的,在Li等[65]最近的一项研究中,发现将氧化石墨烯涂在钛合金上显著促进了骨的形成,这可能归因于石墨烯的优异表面活性,增加了对生长因子的吸附能力。La等[60]比较了单纯氧化石墨烯和氧化石墨烯/铜复合物对成骨诱导的影响,发现氧化石墨烯与铜复合后能够增强碱性磷酸酶和骨钙素的表达,并且还发现氧化石墨烯/铜通过Erk1/2信号通路促进Hif-1α的活性,引起血管内皮生长因子和骨形态发生蛋白2因子表达上调,促进骨的生成。石墨烯及其衍生物与不同物质构成的复合支架相关研究,见表1。"
| [1] Fan Z,Wang J,Wang Z,et al.One-pot synthesis of graphene/ hydroxyapatite nanorod composite for tissue engineering. Carbon. 2014;66(1):407-416.[2] Novoselov KS,Geim AK,Morozov SV,et al.Electric field effect inatomically thin carbon films. Science. 2004;306(5696):666-669.[3] Lee C,Wei X,Kysar JW,et al.Measurement of the elastic properties and intrinsic strength of monolayer grapheme. Science. 2008;321(5887):385-388.[4] Balandin AA,Ghosh S,Bao W,et al.Superior thermal conductivityof single-layer grapheme.Nano Lett. 2008;8(3):902-907.[5] Zhang B,Wang Y,Zhai G,et al.Biomedical applications of thegraphene- based materials.Mater Sci Eng C. 2016;61(8): 953-964.[6] Holt BD,Wright ZM,Arnold AM,et al.Graphene oxide as a scaffold for bone regeneration. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9(3).doi:10.1002/wnan.1437. Epub 2016 Oct 26.[7] Jafari M,Paknejad Z,Rad MR,et al.Polymeric scaffolds in tissue engineering: a literature review. J Biomed Mater Res B Appl Biomater. 2017;105(2):431-459.[8] Gong T,Xie J,Liao J,et al.Nanomaterials and bone regeneration. Bone Res.2015;3(3):123-129.[9] 吕成奇,邹德荣.石墨烯材料在组织工程方面应用的新进展[J].口腔医学,2014,34(4):304-306.[10] Zhang Y,Ali SF,Dervishi E,et al. Cytotoxicity effects of graphene and single-wall carbon nanotubes in neural phaeochromocytoma- derived PC12 cells.Acs Nano. 2010;4(6):3181-3186.[11] Kalbacova M,Broz A,Kong J,et al.Graphene substrates promoteadherence of human osteoblasts and mesenchymal stromal cells.Carbon.2010;48(15):4323-4329.[12] 徐宏杨,范敏敏,张志荣,等.氧化石墨烯PEG化后对L929细胞毒性的影响[J].华西药学杂志,2015, 30(4):425-427.[13] Liao KH,Lin YS,Macosko CW,et al.Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblastsACS Appl Mater Interfaces.2011;3(7):2607-2615.[14] 赵梦,温朝辉.石墨烯及其复合材料在组织工程领域的研究进展[J].医学研究杂志,2017,46(12):175-178.[15] Bengtson S,Kling K,Madsen AM,et al.No cytotoxicity or genotoxicity of graphene and graphene oxide in murine lung epithelial FE1 cells in vitro. Environ Mol Mutagen. 2016;57(6): 469-476.[16] Girish CM,Sasidharan A,Gowd GS,et al.Confocal Raman imaging study showing macrophage mediated biodegradation of graphene in vivo.Adv Healthc Mater.2013;2(11):1489-1500.[17] Schinwald A,Murphy F,Askounis A,et al.Minimal oxidation and inflammogenicity of pristine graphene with residence in the lung. Nanotoxicology.2014;8(8):824-832.[18] Jin L,Lee JH,Jin OS,et al.Stimulated osteogenic differentiation of human mesenchymal stem cells by reduced graphene oxide.J Nanosci Nanotechnol.2015;15(10):7966-7970.[19] Kang ES,Kim DS,Suhito IR,et al.Guiding osteogenesis of mesenchymal stem cells using carbon-based nanomaterials. Nano Convergence.2017;4(1):1-14.[20] Han X,Chua M,Islam I,et al.CVD-grown monolayer graphene induces osteogenic but not odontoblastic differentiation of dental pulp stem cells.Dent Mater.2016;33(1):13-21.[21] Crowder SW,Prasai D,Rath R,et al.Three-dimensional graphene foams promote osteogenic differentiation of humanmesenchymal stem cells. Nanoscale. 2013;5(10):4171-4176.[22] Lee WC,Lim CH,Shi H,et al.Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. Acs Nano. 2011;5(9):7334-7341.[23] Aryaei A,Jayatissa AH,Jayasuriya AC,et al.The effect of graphene substrate on osteoblast cell adhesion and proliferation. J Biomed Mater Res A.2014;102(9):3282-3290.[24] Elkhenany H,Amelse L,Lafont A,et al.Graphene supports in vitro proliferation and osteogenic differentiation of goat adult mesenchymal stem cells: potential for bone tissue engineering.J Appl Toxicol.2015;35(4):367-374.[25] Lee WC,Lim CH,Shi H,et al.Origin of Enhanced Stem Cell Growth and Differentiation on Graphene and GrapheneOxide. Acs Nano. 2011;5(9):7334-7341.[26] Nayak TR,Andersen H,Makam VS,et al.Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells.ACS Nano. 2011;5(6):4670-4678.[27] 吕成奇,陆家瑜,于佳,等.自撑式石墨烯水凝胶诱导人脂肪干细胞成骨分化的体外研究[J].口腔医学, 2014,34(7):486-491.[28] Liu Y,Chen T,Du F,et al.Single-Layer Graphene Enhances the Osteogenic Differentiation of Human Mesenchymal Stem Cells In Vitro and In Vivo.J Biomed Nanotechnol. 2016;12(6):1270-1284.[29] Kenry,Lee WC,Loh KP,et al.When stem cells meet graphene: Opportunities and challenges in regenerative medicine. Biomaterials. 2018;155:236-250.[30] Xie H,Cao T,Rodríguez-Lozano FJ,et al.Graphene for the development of the next-generation of biocomposites for dental and medical applications. Dent Mater. 2017;33(7):765-774.[31] Kumar S,Azam MD,Raj S,et al.3D scaffold alters cellular response to graphene in a polymer composite for orthopedic applications.J Biomed Mater Res B Appl Biomater. 2015;104(4): 732-749.[32] Feng P,Peng S,Wu P,et al.A nano-sandwich construct built with graphenenanosheets and carbon nanotubes enhances mechanical properties of hydroxyapatite–polyetheretherketone scaffolds.Int J Nanomedicine.2016;28(6):3487-500.[33] Kumar S,Raj S,Kolanthai E,et al.Chemical functionalization of graphene to augment stem cell osteogenesis and inhibit biofilm formation on polymer composites for orthopedic applications.Acs Appl Mater Interfaces.2015;7(5):3237-3252.[34] Kumar S,Raj S,Sarkar K,et al.Engineering a multi-biofunctional composite using poly(ethylenimine) decorated graphene oxide for bone tissue regeneration. Nanoscale.2016;8(12):6820-6836.[35] Duan S,Yang X,Mei F,et al. Enhanced osteogenic differentiation of mesenchymal stem cells on poly(L-lactide) nanofibrous scaffolds containing carbon nanomaterials.J Biomed Mater Res A. 2015; 103(4):1424-1435.[36] Shao W,He J,Sang F,et al.Enhanced bone formation in electrospun poly(l-lactic-co-glycolic acid)-tussah silk fibroin ultrafine nanofiber scaffolds incorporated with graphene oxide. Mater SciEng C.2016;62(6):823-834.[37] Lyu CQ,Lu JY,Cao CH,et al.Induction of osteogenic differentiation of human adipose-derived stem cells by a novel self-supporting graphene hydrogel film and the possible underlying mechanism.ACS Appl Mater Interfaces. 2015;7(36):20245-20254.[38] Nair M,Nancy D,Krishnan AG,et al.Graphene oxide nanoflakes incorporated gelatin–hydroxyapatite scaffolds enhance osteogenic differentiation of human mesenchymal stem cells. Nanotechnology. 2015;26(16):1-10.[39] Xie C,Lu X,Han L,et al.Biomimetic Mineralized Hierarchical Graphene Oxide/Chitosan Scaffolds with Adsorbability for Immobilization of Nanoparticles for Biomedical Applications. ACS Appl Mater Interfaces. 2016;8(3):1707-1717.[40] Xie Y,Li H,Zhang C,et al.Graphene-reinforced calcium silicate coatings for load-bearing implants. Biomed Mater. 2014;9(2):1-7.[41] Shie MY,Chiang WH,Chen IWP,et al.Synergistic acceleration in the osteogenic and angiogenic differentiation of human mesenchymal stem cells by calcium silicate–graphene composites.Mater Sci Eng C.2017;73(4):726-735.[42] Saravanan S,Anjali C,Vairamani M,et al.Scaffolds containing chitosan, gelatin and graphene oxide for bone tissue regeneration in vitro and in vivo.Int J BiolMacromol. 2017;104(Pt B):1975-1985.[43] Zancanela DC,Simão AMS,Francisco CG,et al.Graphene oxide and titanium: synergistic effects on the biomineralization ability of osteoblast cultures.J Mater Sci Mater Med. 2016;27(4):1-9. [44] Ren N,Li J,QiuJ,et al.Growth and accelerated differentiation of mesenchymal stem cells on graphene-oxide-coated titanate with dexamethasone on surface of titanium implants. Dent Mater 2017; 33(7):525-535.[45] Zhang W,Chang Q,Xu L,et al.Graphene Oxide-Copper Nanocomposite-Coated Porous CaP Scaffold for Vascularized Bone Regeneration via Activation of Hif-1α.Adv Healthc Mater. 2016;5(11):1299-1309.[46] Kumar S,Chatterjee K.Strontium eluting graphene hybrid nanoparticles augment osteogenesis in a 3D tissue scaffold. Nanoscale.2014;7(5):2023-2033.[47] Chen J,Zhang X,Cai H,et al.Osteogenic activity and antibacterial effect of zinc oxide/carboxylatedgraphene oxide nanocomposites:Preparation and in vitro evaluation.Colloids Surfaces B Biointerfaces.2016;147(6):397-407.[48] Zhang S,Yang Q,Zhao W,et al.In vitro and in vivo biocompatibility and osteogenesis of graphene-reinforced nanohydroxyapatite polyamide66 ternary biocomposite as orthopedic implant material. Int J Nanomedicine. 2016;11(7):3179-3189.[49] Nayak TR,Andersen H,Makam VS,et al.Graphene for controlled and accelerated osteogenic differentiation of humanmesenchymal stem cells.ACS Nano. 2011;5(6):4670-4678.[50] Wang CH,Guo ZS,Pang F,et al.Effects of Graphene Modification on the Bioactivation of Polyethylene-Terephthalate-Based Artificial Ligaments.ACS Appl Mater Interfaces. 2015;7(28):15263-15276.[51] Liu H,Cheng J,Chen F,et al.Gelatin functionalizedgraphene oxide for mineralizationof hydroxyapatite:biomimetic and in vitroevaluation. Nanoscale.2014;6(10):5315-5322.[52] Nie W,Peng C,Zhou X,et al.Three-dimensional porous scaffold by self-assembly of reduced graphene oxide andnano-hydroxyapatite composites for bone tissue engineering. Carbon N Y.2017;116(5): 325-337.[53] Lee JH,Shin YC,Jin OS,et al.Reduced graphene oxide-coated hydroxyapatite composites stimulate spontaneous osteogenic differentiation of human mesenchymal stem cells. Nanoscale. 2015;7(6):11642-11651.[54] Lee JH,Shin YC,Lee SM,et al.Enhanced Osteogenesis by Reduced Graphene Oxide/Hydroxyapatite Nanocomposites. Sci Rep.2016;5(5):1-12.[55] Oyefusi A,Olanipekun O,Neelgund GM,et al.Hydroxyapatite grafted carbon nanotubes and graphenenanosheets: Promising bone implant materials. Spectrochim Acta A Mol Biomol Spectrosc. 2014;132(6):410-416.[56] Tatavarty R,Ding H,Lu G,et al.Synergistic acceleration in the osteogenesis of human mesenchymal stem cells bygraphene oxide-calcium phosphate nanocomposites.Chem Commun. 2014; 50(4):8484-8487.[57] Li M,Wang Y,Liu Q,et al.In situ synthesis and biocompatibility of nano hydroxyapatite on pristine and chitosan functionalizedgraphene oxide.J Mater Chem B. 2013;1:475-484.[58] Dong W,Hou L,Li T,et al.A Dual Role of Graphene Oxide Sheet Deposition on Titanate Nanowire Scaffolds for Osteo-implantation: Mechanical Hardener and Surface Activity Regulator.Sci Rep. 2015;5(6):1-13.[59] Zhou Q,Yang P,Li X,et al.Bioactivity of periodontal ligament stem cells on sodium titanate coated with graphene oxide.Sci Rep. 2016;6(7):193-203.[60] La WG,Park S,Yoon HH,et al.Delivery of a therapeutic protein for bone regeneration from a substrate coated with graphene oxide.Small.2013;9(5):4051-4060.[61] La WG,Kim BS,Jin M,et al.Delivery of bone morphogenetic protein-2 and substance P using graphene oxide for bone regeneration.Int J Nanomedicine.2014;6(5):107-116.[62] Qiu J,Geng H,Wang D,et al.Layer-Number Dependent Antibacterial and Osteogenic Behaviors of Graphene Oxide Electrophoretic Deposited on Titanium.ACS Appl Mater Interfaces. 2017;9(6):12253-12263.[63] Subbiah R,Du P,Van SY,et al.Fibronectin-tethered graphene oxide as an artificial matrix for osteogenesis.Biomed Mater. 2014;9(3): 650-663.[64] Kim SY,Yoo JY,Ohe JY,et al.Differential expression of osteo-modulatory molecules in periodontal ligament stem cells in response to modified titanium surfaces.Biomed Res Int. 2014;9(1): 1-12.[65] Li K,Yan J,Wang C,et al.Graphene modified titanium alloy promote the adhesion, proliferation and osteogenic differentiation of bone marrow stromal cells.BiochemBiophys Res Commun. 2017;489(6):187-192. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [3] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [4] | Zhang Xiumei, Zhai Yunkai, Zhao Jie, Zhao Meng. Research hotspots of organoid models in recent 10 years: a search in domestic and foreign databases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1249-1255. |
| [5] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [6] | Shi Yangyang, Qin Yingfei, Wu Fuling, He Xiao, Zhang Xuejing. Pretreatment of placental mesenchymal stem cells to prevent bronchiolitis in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 991-995. |
| [7] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [8] | Fan Quanbao, Luo Huina, Wang Bingyun, Chen Shengfeng, Cui Lianxu, Jiang Wenkang, Zhao Mingming, Wang Jingjing, Luo Dongzhang, Chen Zhisheng, Bai Yinshan, Liu Canying, Zhang Hui. Biological characteristics of canine adipose-derived mesenchymal stem cells cultured in hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1002-1007. |
| [9] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [10] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [11] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| [12] | Duan Liyun, Cao Xiaocang. Human placenta mesenchymal stem cells-derived extracellular vesicles regulate collagen deposition in intestinal mucosa of mice with colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1026-1031. |
| [13] | Pei Lili, Sun Guicai, Wang Di. Salvianolic acid B inhibits oxidative damage of bone marrow mesenchymal stem cells and promotes differentiation into cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1032-1036. |
| [14] | Guan Qian, Luan Zuo, Ye Dou, Yang Yinxiang, Wang Zhaoyan, Wang Qian, Yao Ruiqin. Morphological changes in human oligodendrocyte progenitor cells during passage [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1045-1049. |
| [15] | Wang Zhengdong, Huang Na, Chen Jingxian, Zheng Zuobing, Hu Xinyu, Li Mei, Su Xiao, Su Xuesen, Yan Nan. Inhibitory effects of sodium butyrate on microglial activation and expression of inflammatory factors induced by fluorosis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1075-1080. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||