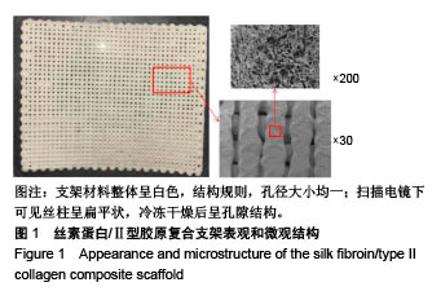
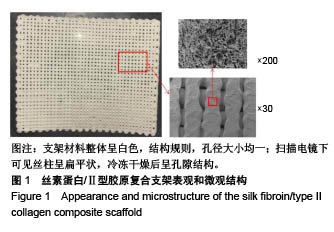
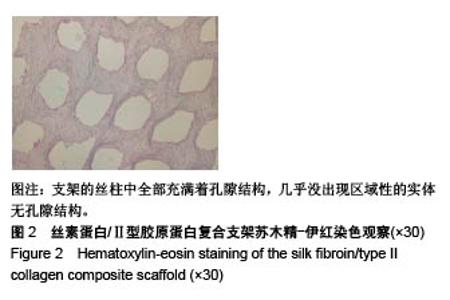
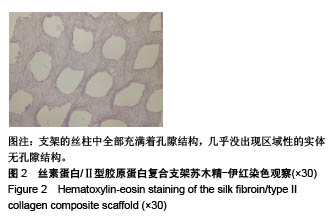
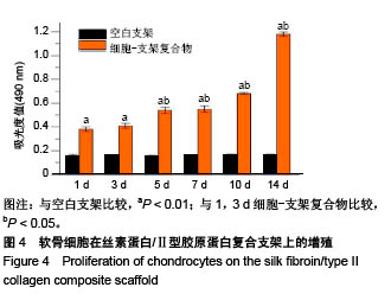
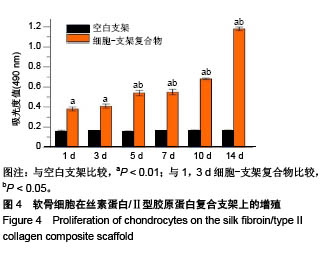
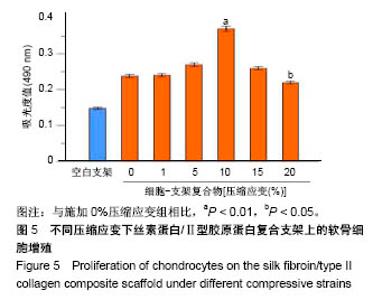
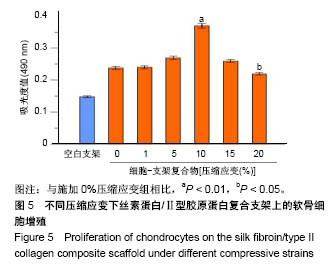
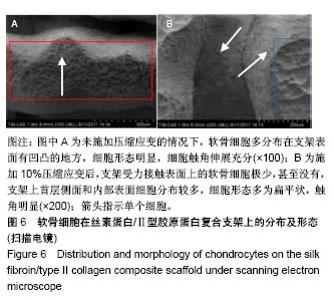
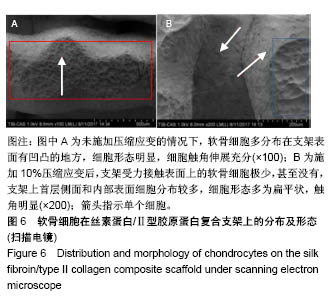
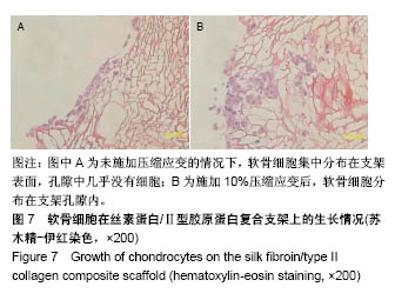
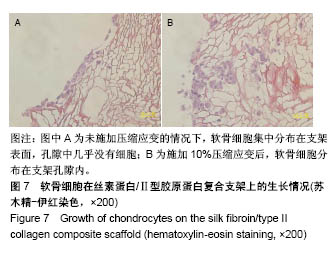
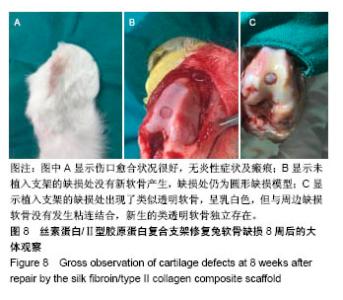
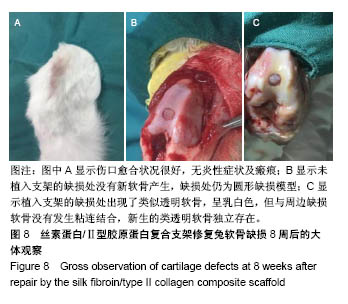
| [1] Hunter W. Of the structure and disease of articulating cartilages.1743. Philos Trans R Soc Lond.1995;42(317):3-6.[2] Jakobsen RB, Engebretsen L, Slauterbeck JR.An analysis of the quality of cartilage repair studies. J Bone Joint Surg Am. 2005; 87(10):2232-2239.[3] Foss C, Merzari E, Migliaresi C, et al. Silk fibroin/hyaluronic acid 3D matrices for cartilage tissue engineering. Biomacromolecules. 2013;14(1):38-47.[4] Aliramaji S, Zamanian A, Mozafari M.Super-paramagnetic responsive silk fibroin/chitosan/magnetite scaffolds with tunable pore structures for bone tissue engineering applications. Mater Sci Eng C. 2017;70(Pt 1):736-744.[5] Li DW, Lei X, He FL, et al.Silk fibroin/chitosan scaffold with tunable properties and low inflammatory response assists the differentiation of bone marrow mesenchymal stem cells. Int J Biol Macromol. 2017;105(Pt 1):584-597.[6] Li DW, He FL, He J, et al.From 2D to 3D: The morphology, proliferation and differentiation of MC3T3-E1 on silk fibroin/chitosan matrices.Carbohydr Polym. 2017;178:69-77.[7] Ghaznavi AM, Kokai LE, Lovett ML, et al. Silk fibroin conduits: a cellular and functional assessment of peripheral nerve repair. Ann Plast Surg.2011; 66(3):273-279.[8] Wang J, Yang Q, Cheng N, et al. Collagen/silk fibroin composite scaffold incorporated with PLGA microsphere for cartilage repair.Mater Sci Eng C.2016;61:705-711.[9] Zhang Q, Yao K, Liu L, et al. Evaluation of porous collagen membrane in guided tissue regeneration. Artif Cells Blood Substit Immobil Biotechnol.1999;27(3):245.[10] Solchaga LA, Temenoff JS, Gao J, et al.Repair of osteochondral defects with hyaluronan- and polyester-based scaffolds.Osteoarthritis Cartilage. 2005;13(4):297-309.[11] Jeon YH, Choi JH, Sung JK, et al.Different effects of PLGA and chitosan scaffolds on human cartilage tissue engineering.J Craniofac Surg.2007;18(6):1249.[12] Nehrer S, Spector M, Minas T. Histologic analysis of tissue after failed cartilage repair procedures.Clin Orthop Relat Res. 1999; 365(365):149-162.[13] Silverman RP, Passaretti D, Huang W, et al.Injectable tissue-engineered cartilage using a fibrin glue polymer. Plast Reconstr Surg.1999;103(7):1809.[14] Gao L, Yuan Q, Li R, et al.Theoretical Prediction and Experimental Testing of Mechanical Properties for 3D Printed Silk Fibroin-Type II Collagen Scaffolds for Cartilage Regeneration. 2018.[15] Widmer MS, Mikos AG. Fabrication of Biodegradable Polymer Scaffolds for Tissue Engineering// Frontiers in Tissue Engineering,1998:107-120.[16] 夏友谊.纳米二氧化钛-丝素复合膜的制备及其光催化甲基橙的研究[J].现代化工,2005,25(2):41-43.[17] Guziewicz N, Best A, Perezramirez B, et al. Lyophilized silk fibroin hydrogels for the sustained local delivery of therapeutic monoclonal antibodies. Biomaterials. 2011; 32(10):2642-2650.[18] Rockwood DN, Preda RC, Yücel T, et al.Materials fabrication from Bombyx mori silk fibroin. Nat Protoc. 2011; 6(10):1612-31.[19] Fratzl P. Collagen : structure and mechanics[M]// Collagen. Springer US, 2008:1-13.[20] 周惠琼,吴东海,李东民,等.应用酶解及氯化钠盐析方法对4个种属Ⅱ型胶原的提纯及比较[J].中华医学杂志, 2001, 81(11): 696-697.[21] 成晓瑜,陈文华,裴显庆,等.高纯度牛骨胶原的制备及其结构表征[J].食品科学,2009,30(7):29-32.[22] Yoo DJ.Computer-aided porous scaffold design for tissue engineering using triply periodic minimal surfaces.Int J Precis Eng Manufac.2011; 12(1): 61-71.[23] Mullen CA, Haugh MG, Schaffler MB, et al.Osteocyte differentiation is regulated by extracellular matrix stiffness and intercellular separation.J Mech Behav Biomed Mater. 2013; 28(4):183-194.[24] Chen DC, Lai YL, Lee SY, et al. Osteoblastic response to collagen scaffolds varied in freezing temperature and glutaraldehyde crosslinking. J Biomed Mater Res A. 2010; 80A(2):399-409.[25] Farrell E, O'Brien F J, Doyle P, et al. A collagen-glycosaminoglycan scaffold supports adult rat mesenchymal stem cell differentiation along osteogenic and chondrogenic routes.Tissue Eng. 2006;12(3):459.[26] Clarke B. Normal bone anatomy and physiology.Clin J Am Soc Nephrol.2008;3 Suppl 3(supplement 3):S131.[27] Sanz-Herrera JA, García-Aznar JM, DoblarÉ M. Micro–macro numerical modelling of bone regeneration in tissue engineering. Comput Methods Appl Mech Eng.2008; 197(33):3092-3107. |