Chinese Journal of Tissue Engineering Research ›› 2018, Vol. 22 ›› Issue (33): 5373-5378.doi: 10.3969/j.issn.2095-4344.0672
Previous Articles Next Articles
Mesenchymal stem cells for treating osteogenesis imperfecta: directly differentiating into functional cells or functioning via paracrine mechanism?
Wang Zi-han, Liu Yi, Ju Ming-yan, Li Guang
- Department of Genetics, School of Basic Medicine, Tianjin Medical University, Tianjin 300070, China
-
Revised:2018-08-31Online:2018-11-28Published:2018-11-28 -
Contact:Li Guang, Professor, Doctoral supervisor, Department of Genetics, School of Basic Medicine, Tianjin Medical University, Tianjin 300070, China -
About author:Wang Zi-han, Department of Genetics, School of Basic Medicine, Tianjin Medical University, Tianjin 300070, China -
Supported by:the National Key R&D Program of China, No. 2017YFC1001904; Tianjin City Science and Technology Support Program, No. 16YFZCSY00900
CLC Number:
Cite this article
Wang Zi-han, Liu Yi, Ju Ming-yan, Li Guang. Mesenchymal stem cells for treating osteogenesis imperfecta: directly differentiating into functional cells or functioning via paracrine mechanism?[J]. Chinese Journal of Tissue Engineering Research, 2018, 22(33): 5373-5378.
share this article
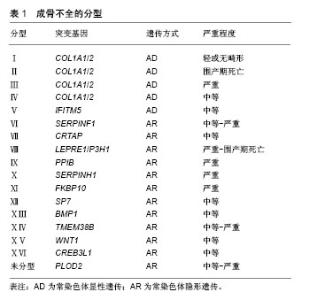
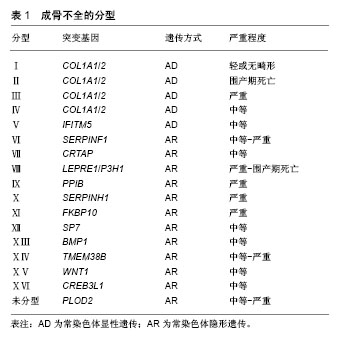
2.1 成骨不全概述 成骨不全又称脆骨症,发病率为1/20 000-1/15 000[8]。作为一种全身性结缔组织疾病,成骨不全主要累及骨骼系统,使骨骼脆性增加,反复骨折,骨关节进行性畸形,同时也可累及眼、耳、皮肤、牙齿等,出现蓝巩膜、耳聋、皮肤异常以及牙本质发育不全等临床症状[1]。临床约90%的成骨不全患者由编码Ⅰ型胶原蛋白的COL1A1/COL1A2基因突变所致,主要呈常染色体显性遗传[9]。基于临床和影像学特征,1979年Sillence等[5]将成骨不全分为Ⅰ-Ⅳ 型,随着新的表型和致病基因的陆续发现,后续研究者又在此基础上将成骨不全扩充至ⅩⅥ型[8](表1),但Sillence分型依然是目前临床应用最广的分型方法[9]。其中,Ⅰ型(轻型)成骨不全仅与胶原的数量减少相关,Ⅱ型(致死型/先天型)成骨不全患者常于宫内死亡或在出生后短期内死亡[10]、Ⅲ型(重型)和Ⅳ型(中型)均存在不同程度的胶原结构改变[1]。"
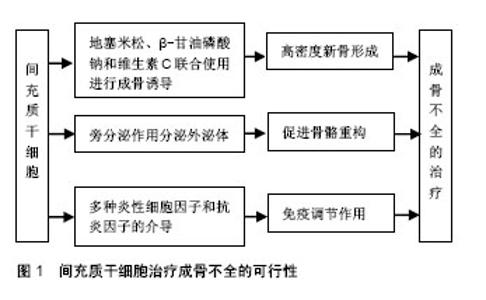
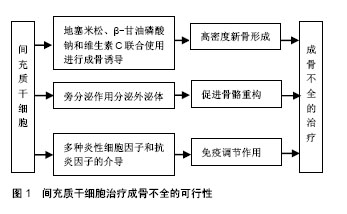
正常骨的生长发育,需要成骨细胞的骨形成作用和破骨细胞的骨吸收作用共同维持,成骨与破骨的平衡是维持正常骨量的关键。成骨细胞是骨形成的主要功能细胞,负责骨基质的合成、分泌和矿化[2]。Ⅰ型胶原是骨基质中含量最多(约占90%)的有机成分,由2条COL1A1基因编码的α1链和1条COL1A2基因编码的α2链构成,α1链和α2链中含有甘氨酸(glycine,Gly)三联体的Gly-X-Y重复序列(X和Y多为脯氨酸和羟脯氨酸),形成稳定的三股螺旋结构[8]。胶原分子之间通过共价键横向交联,构成钙和磷等矿物质沉积的矿化骨架[8]。COL1A1/COL1A2基因中任何一个位点发生突变都有可能使成骨细胞合成胶原的结构或量发生异常改变,进而引起矿化异常,导致成骨不全发生。目前报道的COL1A1/COL1A2基因突变包括无义突变、错义突变、移码突变、剪接突变等类型,其中无义突变、移码突变、剪接突变主要使胶原数量减少,其患者临床症状较轻,通常为Ⅰ型;错义突变主要导致三联体中甘氨酸被丝氨酸为主的其他氨基酸替代,引起Ⅰ型胶原蛋白三螺旋区域空间构象改变,其患者临床表型较重,主要为Ⅱ-Ⅳ型(Ⅱ型常于宫内死亡或在出生后短期内死亡,临床不常见)[11]。 2.2 成骨不全的传统治疗方法 目前,临床上针对成骨不全主要有药物治疗和外科手术治疗等方法。 成骨不全的药物治疗中,双膦酸盐类是目前临床治疗成骨不全最有效的药物[2],可以提高骨密度、降低骨折发生风险、减轻骨痛、增强肌力、改善生活质量等[12-15],但不同种类双膦酸盐的用药剂量不同,对成骨不全患者的疗效也不同,仍有待进一步研究[14],且长期使用会出现颌骨坏死、食管癌、严重肌肉骨骼痛和肾功能衰竭等严重不良反应[15]。除双膦酸盐类之外,还有一些药物研究主要以抑制破骨和调节成骨合成代谢为主,包括生长激素、甲状旁腺激素、骨硬化蛋白抗体等。生长激素可直接作用于成骨细胞[16],促进Ⅰ型胶原的合成和长骨的生长发育[17],但同时增加骨转换率,使成骨不全患者存在畸形加重的风险[18]。甲状旁腺激素可调节哺乳动物钙磷代谢[19],激活休眠的骨内膜细胞并促进其分化为成骨细胞,使成骨细胞的数目显著增加,进而增加骨形成率,提高患者骨量和骨密度[20],但能否作为成骨不全的一线用药仍需进一步研究。骨硬化蛋白抗体可以靶向抑制骨硬化蛋白[21],促进骨形成,提高骨量[22],被广泛用于骨质疏松症的研究,治疗胶原蛋白翻译后修饰缺陷引起的成骨不全效果显著[23],但仍处于动物实验阶段[24],临床治疗成骨不全的研究未见报道。 成骨不全的外科手术治疗主要集中在长骨畸形及反复骨折、脊柱侧凸和听力缺失的矫正与治疗等。针对长骨畸形、移位和不稳定性骨折,除常规的钢板固定和骨切开术外,还可通过微创手术在长骨骨髓腔内放置可伸缩髓内钉,不仅无需进行关节切除就可稳定严重骨折,而且在矫正治疗中断后仍能提供持续的内部支撑[3]。尽管可伸缩髓内钉可降低移位率,但手术操作难度大,术后并发症较多,易增加成骨不全患者再骨折风险[25]。脊柱侧凸是成骨不全的常见并发症之一,常引起疼痛和活动障碍,严重时亦可危及生命。当成骨不全患者脊柱稳定且Cobb角> 50°时,可采用halo式支架以防止侧凸的持续加重[26],但其应用可能会导致脊柱韧带松弛,使曲线矫正的效果逐渐丧失[27]。成骨不全也常伴随听力缺失的发生,在成骨不全患者听力缺失初期可使用助听器进行治疗,但随着病情的不断加重,患者则需进行镫骨切除术治疗[28]。 2.3 MSCs治疗成骨不全的可行性(图1)"
| [1] Marini JC, Forlino A, Bächinger HP, et al. Osteogenesis imperfecta. Nat Rev Dis Primers. 2017;3:17052. [2] Morello R. Osteogenesis imperfecta and therapeutics. Matrix Biol. 2018 Mar 11. doi:10.1016/j. matbio. 2018.03.010. [Epub ahead of print][3] Esposito P, Plotkin H. Surgical treatment of osteogenesis imperfecta: current concepts. Curr Opin Pediatr. 2008;20(1): 52-57. [4] 李颖,董武.干细胞治疗女性压力性尿失禁研究进展[J].中国实用妇科与产科杂志,2011,27(5):398-400.[5] Sillence DO, Rimoin DL, Danks DM. Clinical variability in osteogenesis imperfecta-variable expressivity or genetic heterogeneity. Birth Defects Orig Artic Ser. 1979;15(5B): 113-129. [6] Friedenstein AJ, Deriglasova UF, Kulagina NN, et al. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2(2):83-92. [7] Horwitz EM, Prockop DJ, Fitzpatrick LA, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5(3):309-313. [8] Forlino A, Marini JC. Osteogenesis imperfecta. Lancet. 2016;387(10028):1657-1671. [9] Forlino A, Cabral WA, Barnes AM, et al. New perspectives on osteogenesis imperfecta. Nat Rev Endocrinol. 2011;7(9): 540-557. [10] Marini JC, Blissett AR. New genes in bone development: what's new in osteogenesis imperfecta. J Clin Endocrinol Metab. 2013;98(8):3095-3103. [11] Marini JC, Forlino A, Cabral WA, et al. Consortium for osteogenesis imperfecta mutations in the helical domain of type I collagen: regions rich in lethal mutations align with collagen binding sites for integrins and proteoglycans. Hum Mutat. 2007;28(3):209-221. [12] Castillo H, Samson-Fang L, American Academy for Cerebral Palsy and Developmental Medicine Treatment Outcomes Committee Review Panel. Effects of bisphosphonates in children with osteogenesis imperfecta: an AACPDM systematic review. Dev Med Child Neurol. 2009;51(1):17-29. [13] Semler O, Beccard R, Palmisano D, et al. Reshaping of vertebrae during treatment with neridronate or pamidronate in children with osteogenesis imperfecta. Horm Res Paediatr. 2011;76(5):321-327. [14] Gök?en D, Coker M, Darcan S, et al. Low-dose intravenous pamidronate treatment in osteogenesis imperfecta. Turk J Pediatr. 2006;48(2):124-129. [15] Barros ER, Saraiva GL, de Oliveira TP, et al. Safety and efficacy of a 1-year treatment with zoledronic acid compared with pamidronate in children with osteogenesis imperfecta. J Pediatr Endocrinol Metab. 2012;25(5-6):485-491. [16] Marini JC, Hopkins E, Glorieux FH, et al. Positive linear growth and bone responses to growth hormone treatment in children with types III and IV osteogenesis imperfecta: high predictive value of the carboxyterminal propeptide of type I procollagen. J Bone Miner Res. 2003;18(2):237-243. [17] Antoniazzi F, Monti E, Venturi G, et al. GH in combination with bisphosphonate treatment in osteogenesis imperfecta. Eur J Endocrinol. 2010;163(3):479-487. [18] Florenzano P, Ernst D, Lustig N, et al. Vitamin D and parathyroid hormone levels and bone mineral density in patients undergoing hematopoietic cell transplantation. Rev Med Chil. 2016;144(9):1119-1124. [19] Välimäki VV, Mäkitie O, Pereira R, et al. Teriparatide Treatment in Patients With WNT1 or PLS3 Mutation-Related Early-Onset Osteoporosis: A Pilot Study. J Clin Endocrinol Metab. 2017;102(2):535-544. [20] Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19(2):179-192. [21] Li X, Ominsky MS, Warmington KS, et al. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res. 2009;24(4):578-588. [22] Li X, Warmington KS, Niu QT, et al. Inhibition of sclerostin by monoclonal antibody increases bone formation, bone mass, and bone strength in aged male rats. J Bone Miner Res. 2010;25(12):2647-2656. [23] Grafe I, Alexander S, Yang T, et al. Sclerostin Antibody Treatment Improves the Bone Phenotype of Crtap(-/-) Mice, a Model of Recessive Osteogenesis Imperfecta. J Bone Miner Res. 2016;31(5):1030-1040. [24] Veverka V, Henry AJ, Slocombe PM, et al. Characterization of the structural features and interactions of sclerostin: molecular insight into a key regulator of Wnt-mediated bone formation. J Biol Chem. 2009;284(16):10890-10900. [25] Cho TJ, Kim JB, Lee JW, et al. Fracture in long bones stabilised by telescopic intramedullary rods in patients with osteogenesis imperfecta. J Bone Joint Surg Br. 2011;93(5): 634-638. [26] Monti E, Mottes M, Fraschini P, et al. Current and emerging treatments for the management of osteogenesis imperfecta. Ther Clin Risk Manag. 2010;6:367-381. [27] Janus GJ, Finidori G, Engelbert RH, et al. Operative treatment of severe scoliosis in osteogenesis imperfecta: results of 20 patients after halo traction and posterior spondylodesis with instrumentation. Eur Spine J. 2000;9(6):486-491. [28] Swinnen FK, De Leenheer EM, Coucke PJ, et al. Stapes surgery in osteogenesis imperfecta: retrospective analysis of 34 operated ears. Audiol Neurootol. 2012;17(3):198-206. [29] Green DR, Levine B. To be or not to be? How selective autophagy and cell death govern cell fate. Cell. 2014;157(1): 65-75. [30] Da Silva Meirelles L, Malta TM, Panepucci RA, et al. Transcriptomic comparisons between cultured human adipose tissue-derived pericytes and mesenchymal stromal cells. Genom Data. 2015;7:20-25. [31] Li F, Wang X, Niyibizi C. Bone marrow stromal cells contribute to bone formation following infusion into femoral cavities of a mouse model of osteogenesis imperfecta. Bone. 2010;47(3): 546-555. [32] Lee CW, Huang WC, Huang HD, et al. DNA Methyltransferases Modulate Hepatogenic Lineage Plasticity of Mesenchymal Stromal Cells. Stem Cell Reports. 2017;9(1): 247-263. [33] Lotfy A, Salama M, Zahran F, et al. Characterization of mesenchymal stem cells derived from rat bone marrow and adipose tissue: a comparative study. Int J Stem Cells. 2014; 7(2):135-142. [34] Chen R, Mian M, Fu M, et al. Attenuation of the progression of articular cartilage degeneration by inhibition of TGF-β1 signaling in a mouse model of osteoarthritis. Am J Pathol. 2015;185(11):2875-2885. [35] Kaneto CM, Lima PS, Zanette DL, et al. Osteoblastic differentiation of bone marrow mesenchymal stromal cells in Bruck Syndrome. BMC Med Genet. 2016;17(1):38. [36] Dueñas F, Becerra V, Cortes Y, et al. Hepatogenic and neurogenic differentiation of bone marrow mesenchymal stem cells from abattoir-derived bovine fetuses. BMC Vet Res. 2014;10:154. [37] Boyiadzis M, Whiteside TL. Information transfer by exosomes: A new frontier in hematologic malignancies. Blood Rev. 2015; 29(5):281-290. [38] Lee Y, El Andaloussi S, Wood MJ. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet. 2012;21(R1): R125-134. [39] Otsuru S, Desbourdes L, Guess AJ, et al. Extracellular vesicles released from mesenchymal stromal cells stimulate bone growth in osteogenesis imperfecta. Cytotherapy. 2018; 20(1):62-73. [40] Yang Y, Adachi K, Sheridan MA, et al. Heightened potency of human pluripotent stem cell lines created by transient BMP4 exposure. Proc Natl Acad Sci U S A. 2015;112(18): E2337-2346. [41] Samsonraj RM, Raghunath M, Nurcombe V, et al. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Transl Med. 2017;6(12):2173-2185. [42] Rousseau J, Gioia R, Layrolle P, et al. Allele-specific Col1a1 silencing reduces mutant collagen in fibroblasts from Brtl mouse, a model for classical osteogenesis imperfecta. Eur J Hum Genet. 2014;22(5):667-674. [43] English K, Mahon BP. Allogeneic mesenchymal stem cells: agents of immune modulation. J Cell Biochem. 2011;112(8): 1963-1968. [44] Jones GN, Moschidou D, Lay K, et al. Upregulating CXCR4 in human fetal mesenchymal stem cells enhances engraftment and bone mechanics in a mouse model of osteogenesis imperfecta. Stem Cells Transl Med. 2012;1(1):70-78. [45] Guillot PV, Abass O, Bassett JH, et al. Intrauterine transplantation of human fetal mesenchymal stem cells from first-trimester blood repairs bone and reduces fractures in osteogenesis imperfecta mice. Blood. 2008;111(3): 1717-1725. [46] Vanleene M, Saldanha Z, Cloyd KL, et al. Transplantation of human fetal blood stem cells in the osteogenesis imperfecta mouse leads to improvement in multiscale tissue properties. Blood. 2011;117(3):1053-1060. [47] Horwitz EM, Prockop DJ, Gordon PL, et al. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood. 2001;97(5):1227-1231. [48] Otsuru S, Gordon PL, Shimono K, et al. Transplanted bone marrow mononuclear cells and MSCs impart clinical benefit to children with osteogenesis imperfecta through different mechanisms. Blood. 2012;120(9):1933-1941. [49] Horwitz EM, Gordon PL, Koo WK, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci U S A. 2002;99(13):8932-8937. [50] Le Blanc K, Götherström C, Ringdén O, et al. Fetal mesenchymal stem-cell engraftment in bone after in utero transplantation in a patient with severe osteogenesis imperfecta. Transplantation. 2005;79(11):1607-1614. [51] Götherström C, Westgren M, Shaw SW, et al. Pre- and postnatal transplantation of fetal mesenchymal stem cells in osteogenesis imperfecta: a two-center experience. Stem Cells Transl Med. 2014;3(2):255-264. [52] Chamberlain JR, Deyle DR, Schwarze U, et al. Gene targeting of mutant COL1A2 alleles in mesenchymal stem cells from individuals with osteogenesis imperfecta. Mol Ther. 2008;16(1):187-193. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [3] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [4] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [5] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [6] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [7] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [8] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [9] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [10] | Xing Hao, Zhang Yonghong, Wang Dong. Advantages and disadvantages of repairing large-segment bone defect [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 426-430. |
| [11] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| [12] | Wang Hao, Chen Mingxue, Li Junkang, Luo Xujiang, Peng Liqing, Li Huo, Huang Bo, Tian Guangzhao, Liu Shuyun, Sui Xiang, Huang Jingxiang, Guo Quanyi, Lu Xiaobo. Decellularized porcine skin matrix for tissue-engineered meniscus scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3473-3478. |
| [13] | Mo Jianling, He Shaoru, Feng Bowen, Jian Minqiao, Zhang Xiaohui, Liu Caisheng, Liang Yijing, Liu Yumei, Chen Liang, Zhou Haiyu, Liu Yanhui. Forming prevascularized cell sheets and the expression of angiogenesis-related factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3479-3486. |
| [14] | Liu Chang, Li Datong, Liu Yuan, Kong Lingbo, Guo Rui, Yang Lixue, Hao Dingjun, He Baorong. Poor efficacy after vertebral augmentation surgery of acute symptomatic thoracolumbar osteoporotic compression fracture: relationship with bone cement, bone mineral density, and adjacent fractures [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3510-3516. |
| [15] | Liu Liyong, Zhou Lei. Research and development status and development trend of hydrogel in tissue engineering based on patent information [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3527-3533. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||