Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (14): 2234-2240.doi: 10.3969/j.issn.2095-4344.2017.14.017
Previous Articles Next Articles
In vitro degradation of concentrated growth factor fibrin versus platelet-rich fibrin
Li Yong-bin1, Sun Ying-chun2, Wei Rong-zhi1, Yang Jian1, Sheng Hai-ying1, Chen Jing1
- 1 Tongzhou Xinhua Hospital of Beijing, Beijing 101100, China; 2 Stomatology Hospital of Tianjin Medical University, Tianjin 300070, China
-
Received:2017-01-21Online:2017-05-18Published:2017-06-10 -
Contact:Sun Ying-chun, Chief physician, Stomatology Hospital of Tianjin Medical University, Tianjin 300070, China -
About author:Li Yong-bin, Master, Associate chief physician, Tongzhou Xinhua Hospital of Beijing, Beijing 101100, China
CLC Number:
Cite this article
Li Yong-bin, Sun Ying-chun, Wei Rong-zhi, Yang Jian, Sheng Hai-ying, Chen Jing. In vitro degradation of concentrated growth factor fibrin versus platelet-rich fibrin[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(14): 2234-2240.
share this article
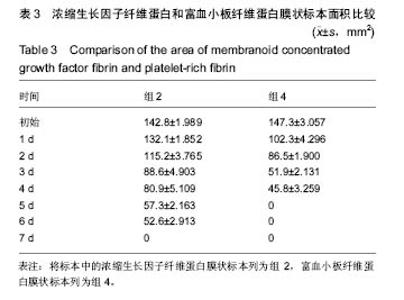
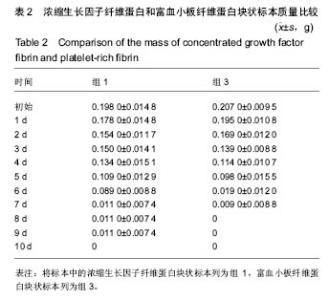
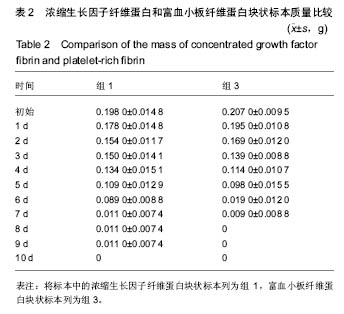
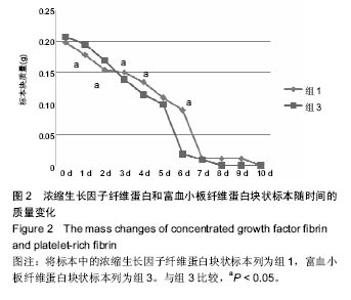
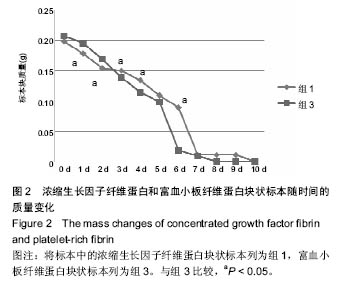
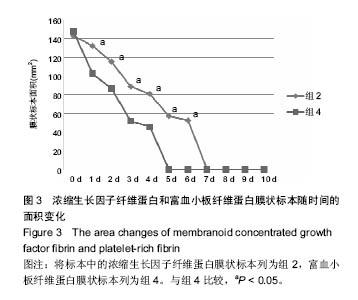
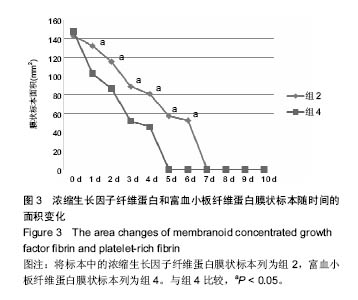
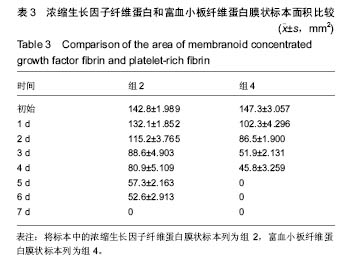
组内进一步方差分析比较结果显示:浓缩生长因子纤维蛋白质量在实验前7 d,除第3天与第2天相比表现为平缓下降,其余时间点均表现为显著下降(P < 0.05);从第8天开始,浓缩生长因子纤维蛋白质量残余量很少,同时维持不变(P > 0.05);第10天浓缩生长因子纤维蛋白完全降解。富血小板纤维蛋白质量在实验前6 d随实验周期的推移表现显著下降(P < 0.05),第7天时保持与第6天持平,第8天降解完全。 组间进一步t 检验比较结果显示:组1与组3中标本块的初始质量差异无显著性意义(P > 0.05);实验开始后,浓缩生长因子纤维蛋白与富血小板纤维蛋白在实验第5天标本块的质量差异无显著性意义(P > 0.05),其余时间点两者质量均表现为差异有显著性意义(P < 0.05)。 实验标本种类与实验周期之间存在交互作用(F=36.071,P <0.05)。 2.2 浓缩生长因子纤维蛋白和富血小板纤维蛋白膜状标本面积比较 见表3及图3。 膜状标本重复测量方差分析组内比较结果显示:随着时间的变化,组2与组4中膜状标本的面积都发生了明显的变化(F=9 750.945,P < 0.05)。组间比较结果显示,浓缩生长因子纤维蛋白与富血小板纤维蛋白膜状标本在相同时间点剩余面积差异有显著性意义(F=3 369.460, P < 0.05)。 组内进一步方差分析比较结果显示:浓缩生长因子纤维蛋白膜状标本面积在实验前6 d表现为显著下降(P < 0.05);实验第7天,浓缩生长因子纤维蛋白完全降解。富血小板纤维蛋白膜状标本面积在实验前4 d随实验周期的推移表现为显著下降(P < 0.05),第5天时降解完全。 组间进一步t 检验比较结果显示:组2与组4中膜状标本的初始面积差异无显著性意义(P > 0.05);实验开始后,浓缩生长因子纤维蛋白与富血小板纤维蛋白膜状标本剩余面积表现为差异有显著性意义(P < 0.05)。 实验膜状标本种类与实验周期之间存在交互作用(F=446.457,P < 0.05)。 "
| [1]Urban IA, Jovanovic SA, Lozada JL, et al. Vertical ridge augmentation using guided bone regeneration (GBR) in three clinical scenarios prior to implant placement: a retrospective study of 35 patients 12 to 72 months after loading. Int J Oral Maxillofac Implants. 2009;24:502-510.[2]Chang NJ, Jhung YR, Issariyakul N, et al. Synergistic stimuli by hydrodynamic pressure and hydrophilic coating on PLGA scaffolds for extracellular matrix synthesis of engineered cartilage. J Biomater Sci Polym Ed.2012;23: 2133-2151.[3]Schar MO, Diaz-Romero Kohl JS. Platelet-rich concentrates differentially release growth factors and induce cell migration in vitro. Clin Orthop Relat Res. 2015;473:1635-1643.[4]Song G, Wu Y, Wang F, et al. Development and preparation of a low-immunogenicity porcine dermal scaffold and its biocompatibility assessment. J Mater Sci Mater Med. 2015; 26:170.[5]Albanese A, Licata ME, Polizzi B, et al. Platelet-rich plasma (PRP) in dental and oral surgery: from the wound healing to bone regeneration. Immun Ageing. 2013;10:23.[6]郝伟,姜明,王新,等. 重组骨形态发生蛋白2、碱性成纤维细胞生长子共转染骨髓间充质干细胞复合纳米羟基磷灰石/重组类人胶原基/聚乳酸复合支架材料支架修复桡骨缺损研究[J].中华实验外科杂志,2013,30(5):1016-1019.[7]Gao C, Harvey EJ, Chua M, et al. MSC-seeded dense collagen scaffolds with a bolus dose of VEGF promote healing of large bone defects. Eur Cell Mater.2013; 26:195-207.[8]Gothard D, Smith EL, Kanczler JM, et al. Tissue engineered bone using select growth factors: A comprehensive review of animal studies and clinical translation studies in man. Eur Cell Mater. 2014;28:166-207.[9]Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part III: leucocyte activation: a new feature for platelet concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101: e51-55.[10]Kim JM, Sohn DS, Bae MS, et al. Flapless transcrestal sinus augmentation using hydrodynamic piezoelectric internal sinus elevation with autologous concentrated growth factors alone. Implant Dent.2014;23:168-174.[11]Kim TH, Kim SH, Sandor GK, et al. Comparison of platelet-rich plasma (PRP), platelet-rich fibrin (PRF), and concentrated growth factor (CGF) in rabbit-skull defect healing. Arch Oral Biol. 2014;59:550-558.[12]Marx RE, Carlson ER, Eichstaedt RM, et al. Georgeff, Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638-646.[13]Choukroun J, Diss A, Simonpieri A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006; 101: e56-60.[14]Fennis JP, Stoelinga PJ, Jansen JA. Mandibular reconstruction: a histological and histomorphometric study on the use of autogenous scaffolds, particulate cortico-cancellous bone grafts and platelet rich plasma in goats. Int J Oral Maxillofac Surg.2004;33:48-55.[15]Rodella LF, Favero G, Boninsegna R, et al. Growth factors, CD34 positive cells, and fibrin network analysis in concentrated growth factors fraction. Microsc Res Tech. 2011; 74:772-777.[16]Barsotti MC, Felice F, Balbarini A, et al. Fibrin as a scaffold for cardiac tissue engineering. Biotechnol Appl Biochem.2011; 58:301-310.[17]龚博林,方圆文.浓缩生长因子治疗复发性口腔溃疡的疗效观察[J].中国老年保健医学, 2013, 11(6):48-49.[18]张晓,董福生,任贵云,等.富自体CGF纤维蛋白膜对人牙龈成纤维细胞黏附及增殖的影响[J].现代口腔医学杂志, 2014,28(4): 197-201.[19]安然, 孙迎春, 高平. 富白细胞血小板纤维蛋白与富血小板血浆对比[J].河北医药, 2013,35(20):3148-3150.[20]Luft S, Keilig L, Jager A, et al. In-vitro evaluation of the corrosion behavior of orthodontic brackets. Orthod Craniofac Res. 2009; 12 :43-51.[21]肖悦, 刘天佳. 人工口腔模拟的口腔环境条件[J].国外医学:.口腔医学分册, 2001,28(3):146-148.[22]孙洁. 富血小板纤维蛋白生物学特性的研究[D].天津医科大学, 2010.[23]Mezawa M, Araki S, Takai H, et al. Regulation of human bone sialoprotein gene transcription by platelet-derived growth factor-BB. Gene.2009; 435:80-87.[24]Xiao WD, Zhong ZM, Tang YZ, et al. Repair of critical size bone defects with porous poly(D,L-lactide)/nacre nanocomposite hollow scaffold. Saudi Med J. 2012;33: 601-607.[25]Ohyama Y, Tanaka T, Shimizu T, et al. Runx2/Smad3 complex negatively regulates TGF-beta-induced connective tissue growth factor gene expression in vascular smooth muscle cells. J Atheroscler Thromb. 2012;19:23-35.[26]Krause DS, Fulzele K, Catic A, et al. Differential regulation of myeloid leukemias by the bone marrow microenvironment. Nat Med. 2013;19:1513-1517.[27]Clarke SA, Brooks RA, Lee PT, et al. The effect of osteogenic growth factors on bone growth into a ceramic filled defect around an implant. J Orthop Res. 2004;22:1016-1024.[28]Sheng MH, Lau KH, Baylink DJ, et al. Role of Osteocyte-derived Insulin-Like Growth Factor I in Developmental Growth, Modeling, Remodeling, and Regeneration of the Bone. J Bone Metab. 2014;21:41-54.[29]Hersel U, Dahmen C, Kessler H, et al. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003;24: 4385-4415.[30]De la Riva B, Nowak C, Sanchez E, et al. VEGF-controlled release within a bone defect from alginate/chitosan/PLA-H scaffolds. Eur J Pharm Biopharm. 2009;73:50-58.[31]Zhou FC, Kelley MR, Chiang YH, et al. Three to four-year-old nonpassaged EGF-responsive neural progenitor cells: proliferation, apoptosis, and DNA repair. Exp Neurol. 2000; 164:200-208.[32]Li H, Ji Q, Chen X, et al. Accelerated bony defect healing based on chitosan thermosensitive hydrogel scaffolds embedded with chitosan nanoparticles for the delivery of BMP2 plasmid DNA. J Biomed Mater Res A.2017;105: 265-273.[33]Landesberg R, Burke A, Pinsky D, et al. Activation of platelet-rich plasma using thrombin receptor agonist peptide. J Oral Maxillofac Surg.2005; 63:529-535.[34]Carano RA, Filvaroff EH. Angiogenesis and bone repair. Drug Discov Today. 2003; 8:980-989.[35]Wang EA, Rosen V, D'Alessandro JS, et al. Recombinant human bone morphogenetic protein induces bone formation. Proc Natl Acad Sci U S A.1990; 87:2220-2224.[36]Lim HP, Mercado-Pagan AE, Yun KD, et al. The effect of rhBMP-2 and PRP delivery by biodegradable beta-tricalcium phosphate scaffolds on new bone formation in a non-through rabbit cranial defect model. J Mater Sci Mater Med.2013; 24:1895-1903.[37]Choi BH, Im CJ, Huh JY, et al. Effect of platelet-rich plasma on bone regeneration in autogenous bone graft. Int J Oral Maxillofac Surg. 2004;33:56-59.[38]Aghaloo TL, Moy PK, Freymiller EG, et al. Evaluation of platelet-rich plasma in combination with freeze-dried bone in the rabbit cranium. A pilot study, Clin Oral Implants Res.2005; 16 :250-257.[39]Dohan Ehrenfest DM, Bielecki T, Jimbo R, et al. Do the fibrin architecture and leukocyte content influence the growth factor release of platelet concentrates? An evidence-based answer comparing a pure platelet-rich plasma (P-PRP) gel and a leukocyte- and platelet-rich fibrin (L-PRF). Curr Pharm Biotechnol. 2012;13(7):1145-1152. [40]Lin HY, Zhang WD, Yu YC, et al. [Clinical study of titanium mesh in conjunction with concentrate growth factors to rebuild severe bone defect of anterior maxilla]. Shanghai Kou Qiang Yi Xue. 2016;25(3):352-356. [41]Mirkovi? S, Djurdjevi?-Mirkovi? T, Pugkar T. Application of concentrated growth factors in reconstruction of bone defects after removal of large jaw cysts--the two cases report. Vojnosanit Pregl. 2015;72(4):368-371.[42]Honda H, Tamai N, Naka N, et al.Bone tissue engineering with bone marrow-derived stromal cells integrated with concentrated growth factor in Rattus norvegicus calvaria defect model. J Artif Organs. 2013;16(3):305-315. [43]Varghese MP, Manuel S, Surej Kumar LK. Potential for Osseous Regeneration of Platelet-Rich Fibrin-A Comparative Study in Mandibular Third Molar Impaction Sockets. J Oral Maxillofac Surg. 2017. pii: S0278-2391(17)30120-9. [44]Al-Hamed FS, Tawfik MA, Abdelfadil E, et al. Efficacy of Platelet-Rich Fibrin After Mandibular Third Molar Extraction: A Systematic Review and Meta-Analysis. J Oral Maxillofac Surg. 2017. pii: S0278-2391(17)30107-6. [45]Kim JH, Woo SM, Choi NK, et al. Effect of Platelet-rich Fibrin on Odontoblastic Differentiation in Human Dental Pulp Cells Exposed to Lipopolysaccharide. J Endod. 2017;43(3): 433-438. [46]Dutta SR, Passi D, Singh P, et al. A randomized comparative prospective study of platelet-rich plasma, platelet-rich fibrin, and hydroxyapatite as a graft material for mandibular third molar extraction socket healing. Natl J Maxillofac Surg. 2016; 7(1):45-51. [47]Sezgin Y, Uraz A, Taner IL, et al. Effects of platelet-rich fibrin on healing of intra-bony defects treated with anorganic bovine bone mineral. Braz Oral Res. 2017;31(0):e15. [48]Park JH, Kim JW, Kim SJ. Does the Addition of Bone Morphogenetic Protein 2 to Platelet-Rich Fibrin Improve Healing After Treatment for Medication-Related Osteonecrosis of the Jaw? J Oral Maxillofac Surg. 2016. pii: S0278-2391(16)31215-0. [49]Neiva RF, Gil LF, Tovar N, et al. The Synergistic Effect of Leukocyte Platelet-Rich Fibrin and Micrometer/Nanometer Surface Texturing on Bone Healing around Immediately Placed Implants: An Experimental Study in Dogs. Biomed Res Int. 2016;2016:9507342.[50]马西. 纤维蛋白形成与降解[J].微循环学杂志, 2002,12(4): 42-44.[51]白彭, 叶平, 吴润发, 等. 两种胶原膜暴露于口腔环境中降解作用的对照研究[J]. 中国口腔种植学杂志, 2011,16(1):56-57. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [3] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [4] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [5] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [6] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [7] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [8] | Li Hui, Chen Lianglong. Application and characteristics of bone graft materials in the treatment of spinal tuberculosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 626-630. |
| [9] | Gao Cangjian, Yang Zhen, Liu Shuyun, Li Hao, Fu Liwei, Zhao Tianyuan, Chen Wei, Liao Zhiyao, Li Pinxue, Sui Xiang, Guo Quanyi. Electrospinning for rotator cuff repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 637-642. |
| [10] | Wei Zhoudan, Li Wenjin, Zhu Li, Wang Yu, Zhao Jiaoyang, Chen Yanan, Guo Dong, Hao Min. Platelet-rich fibrin as a material for alveolar ridge preservation significantly reduces the resorption of alveolar bone height and width after tooth extraction: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 643-648. |
| [11] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [12] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [13] | Guan Jian, Jia Yanfei, Zhang Baoxin , Zhao Guozhong. Application of 4D bioprinting in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 446-455. |
| [14] | Liu Jiali, Suo Hairui, Yang Han, Wang Ling, Xu Mingen. Influence of lay-down angles on mechanical properties of three-dimensional printed polycaprolactone scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2612-2617. |
| [15] | Huang Bo, Chen Mingxue, Peng Liqing, Luo Xujiang, Li Huo, Wang Hao, Tian Qinyu, Lu Xiaobo, Liu Shuyun, Guo Quanyi . Fabrication and biocompatibility of injectable gelatin-methacryloyl/cartilage-derived matrix particles composite hydrogel scaffold [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2600-2606. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||