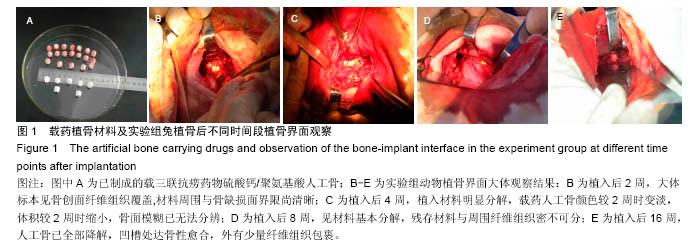
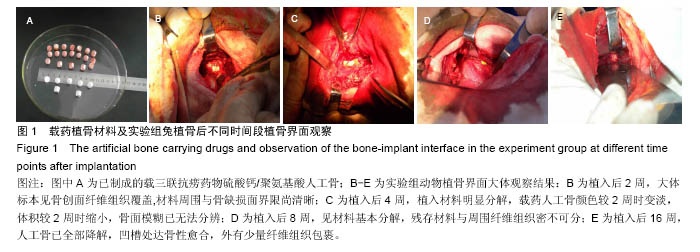
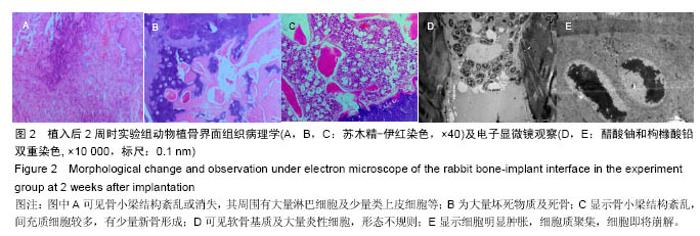
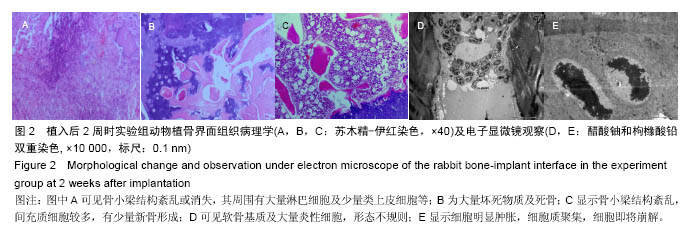
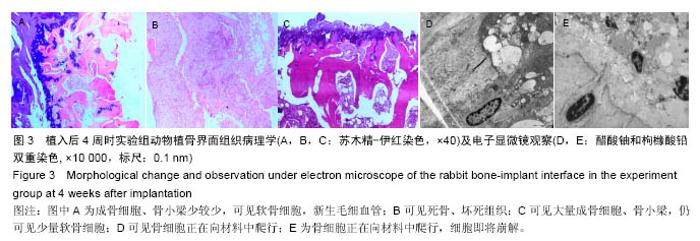
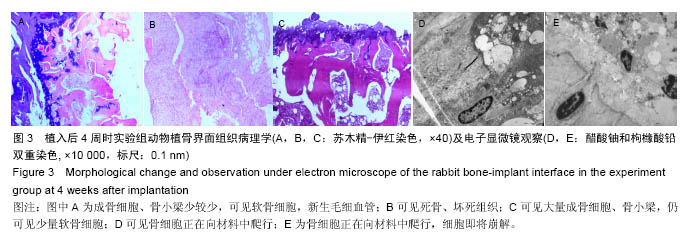
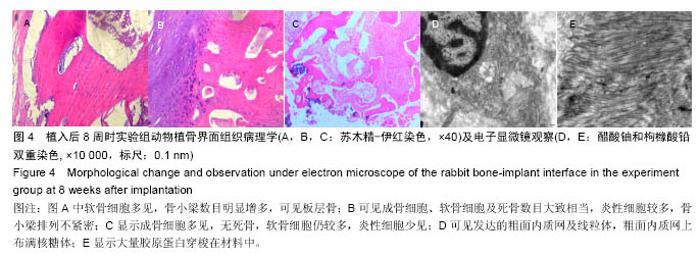
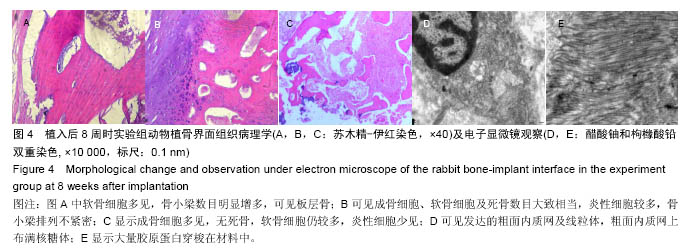
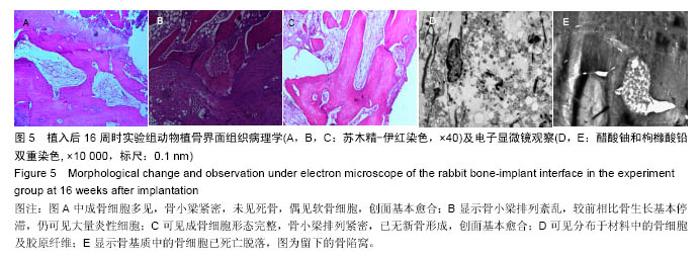
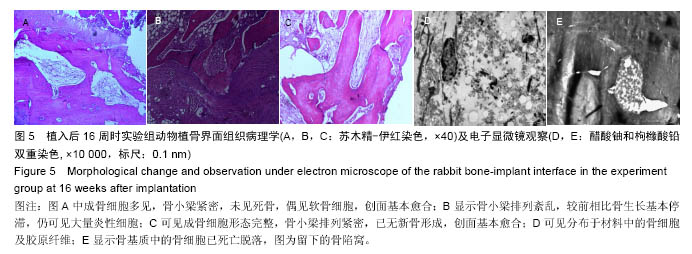
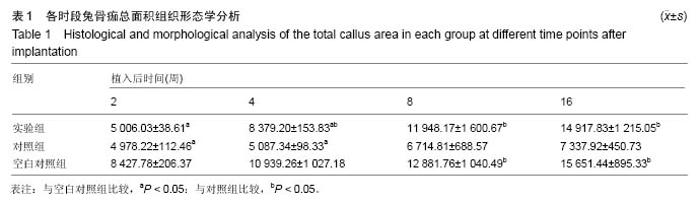
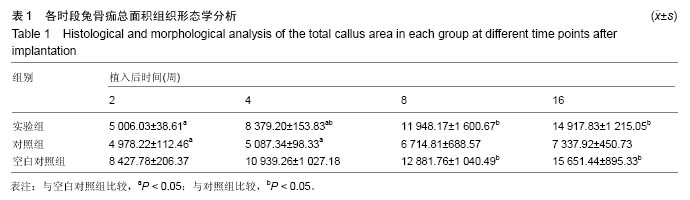
Chinese Journal of Tissue Engineering Research ›› 2016, Vol. 20 ›› Issue (47): 7027-7033.doi: 10.3969/j.issn.2095-4344.2016.47.005
Previous Articles Next Articles
Histological changes of the bone-implant interface after calcium sulfate/ polyaminoacid artificial bone carrying triple-anti-tuberculosis drugs is implanted into the spinal tuberculosis focus
Zhang Zhuo1, Sun Yu-hang2, Geng Guang-qi3, Shi Jian-dang3, Wang Zi-li3, Niu Ning-kui3, Ma Wen-xin3, Liu Hai-tao3, Wang Qian4
- 1 Department of Orthopaedics, Ningxia People’s Hospital, Yinchuan 750001, Ningxia Hui Autonomous Region, China; 2Ningxia Medical University, Yinchuan 750004, Ningxia Hui Autonomous Region, China; 3Department of Spine Surgery, General Hospital of Ningxia Medical University, Yinchuan 750004, Ningxia Hui Autonomous Region, China; 4College of Pharmacy of University of South Florida in U.S, St. Petersburg 33620, U.S