Chinese Journal of Tissue Engineering Research ›› 2016, Vol. 20 ›› Issue (36): 5351-5357.doi: 10.3969/j.issn.2095-4344.2016.36.005
Previous Articles Next Articles
Combination effect of AMD3100 and dexamethasone on the mobilization of hematopoietic stem cells
Yan Bei-zhan, Ma Hui-min, Kong Cun-quan, Liang Yu, Zhu Wei-yan, Jiang Shu-ting
- Department of Blood Transfusion, Henan Provincial People’s Hospital and People’s Hospital of Zhengzhou University, Zhengzhou 450003, Henan Province, China
-
Revised:2016-07-12Online:2016-09-02Published:2016-09-02 -
About author:Yan Bei-zhan, Master, Associate chief technician, Department of Blood Transfusion, Henan Provincial People’s Hospital and People’s Hospital of Zhengzhou University, Zhengzhou 450003, Henan Province, China -
Supported by:the Scientific Tackle Key Project of Henan Province, China, No. 142102310126
CLC Number:
Cite this article
Yan Bei-zhan, Ma Hui-min, Kong Cun-quan, Liang Yu, Zhu Wei-yan, Jiang Shu-ting . Combination effect of AMD3100 and dexamethasone on the mobilization of hematopoietic stem cells[J]. Chinese Journal of Tissue Engineering Research, 2016, 20(36): 5351-5357.
share this article
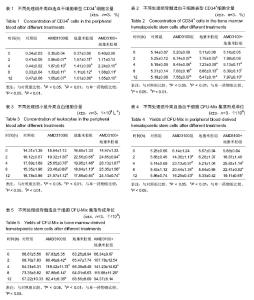
2.1 实验动物数量分析 参加实验60只小鼠,均进入结果分析,中途没有脱落。 2.2 小鼠外周血和骨髓造血干细胞表型CD34+细胞含量 向小鼠体内注射AMD3100或地塞米松后,采集外周血和骨髓造血干细胞,使用流式细胞仪检测CD34+细胞含量,结果见表1和表2。AMD3100和地塞米松均可刺激使外周血CD34+细胞含量上升,分别在4 h和2 h时达到高峰,随后CD34+细胞数量逐渐下降。在骨髓中CD34+细胞含量逐渐上升,与外周血中CD34+细胞含量变化基本一致。当联合使用AMD3100和地塞米松时,外周血和骨髓中CD34+细胞含量上升幅度最大,表明联合使用AMD3100和地塞米松可以促进造血干细胞的动员。 2.3 小鼠外周血中白细胞含量 向小鼠体内注射AMD3100或地塞米松后,小鼠外周血中白细胞含量在 2 h已经开始上升,小鼠注射AMD3100后4 h外周血白细胞含量达到高峰,注射地塞米松2 h后外周血白细胞含量达到高峰,AMD3100和地塞米松联合用药的小鼠外周血白细胞含量在4 h时达到高峰,随后白细胞含量逐渐下降,但依然显著高于对照组,见表3。AMD3100和地塞米松联合用药组外周血白细胞含量高于其他组,表明两者具有协同作用。 2.4 小鼠外周血和骨髓造血干细胞集落形成单位 注射AMD3100的小鼠,外周血和骨髓造血干细胞CFU-Mix集落形成均高于对照组,并且在2 h时集落形成最多,见表4和表5。注射地塞米松的小鼠集落形成数量与对照组无明显差异,联合注射AMD3100和地塞米松形成的集落数高于对照组和单独注射AMD3100的小鼠,表明地塞米松可以加强AMD3100对造血干细胞CFU-Mix集落形成的作用。"
| [1] 王东红. 造血干细胞及其潜能[J]. 卫生职业教育, 2004, 22(6): 121-123. [2] Passweg JR, Halter J, Bucher C, et al. Hematopoietic stem cell transplantation: a review and recommendations for follow-up care for the general practitioner. Swiss Med Wkly. 2012;142:w13696. [3] Nervi B, Link DC, DiPersio JF. Cytokines and hematopoietic stem cell mobilization. J Cell Biochem. 2006;99(3):690-705. [4] 饶彪锋,潘秀英,陆晔,等. AMD3100动员造血干/祖细胞的实验研究[J].江苏医药, 2012,38(5):516-518. [5] Hopman RK, DiPersio JF. Advances in stem cell mobilization. Blood Rev. 2014;28(1):31-40. [6] Guyon A. CXCL12 chemokine and its receptors as major players in the interactions between immune and nervous systems. Front Cell Neurosci. 2014; 8:65. [7] Yang P, Wang G, Huo H, et al. SDF-1/CXCR4 signaling up-regulates survivin to regulate human sacral chondrosarcoma cell cycle and epithelial-mesenchymal transition via ERK and PI3K/AKT pathway. Med Oncol. 2015;32(1):377. [8] Lin CH, Shih CH, Tseng CC, et al. CXCL12 induces connective tissue growth factor expression in human lung fibroblasts through the Rac1/ERK, JNK, and AP-1 pathways. PLoS One. 2014;9(8):e104746. [9] Yu T, Liu K, Wu Y, et al. MicroRNA-9 inhibits the proliferation of oral squamous cell carcinoma cells by suppressing expression of CXCR4 via the Wnt/β-catenin signaling pathway. Oncogene. 2014; 33(42):5017-5027. [10] Zhao S, Wang J, Qin C. Blockade of CXCL12/CXCR4 signaling inhibits intrahepatic cholangiocarcinoma progression and metastasis via inactivation of canonical Wnt pathway. J Exp Clin Cancer Res. 2014; 33:103. [11] Kucia M, Reca R, Miekus K, et al. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23(7):879-894. [12] Sugiyama T, Kohara H, Noda M, et al. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches.Immunity. 2006;25(6):977-988. [13] Ratajczak MZ, Kim CH, Abdel-Latif A, et al. A novel perspective on stem cell homing and mobilization: review on bioactive lipids as potent chemoattractants and cationic peptides as underappreciated modulators of responsiveness to SDF-1 gradients. Leukemia. 2012;26(1):63-72. [14] Wuchter P, Leinweber C, Saffrich R, et al. Plerixafor induces the rapid and transient release of stromal cell-derived factor-1 alpha from human mesenchymal stromal cells and influences the migration behavior of human hematopoietic progenitor cells. Cell Tissue Res. 2014;355(2):315-326. [15] Li J, Hamilton E, Vaughn L, et al. Effectiveness and cost analysis of "just-in-time" salvage plerixafor administration in autologous transplant patients with poor stem cell mobilization kinetics. Transfusion. 2011; 51(10):2175-2182. [16] Tekgündüz E, Altunta? F, S?vg?n S, et al. Plerixafor use in patients with previous mobilization failure: A multicenter experience. Transfus Apher Sci. 2012; 47(1): 77-80. [17] Emir S, Demir HA, Aksu T, et al. Use of plerixafor for peripheral blood stem cell mobilization failure in children. Transfus Apher Sci. 2014;50(2):214-218. [18] Pandey MK, Rastogi S, Kale VP, et al. Targeting CXCL12/CXCR4 Axis in Multiple Myeloma. J Hematol Thrombo Dis 2014; 2:159. [19] Fricker SP. Physiology and pharmacology of plerixafor. Transfus Med Hemother. 2013;40(4):237-245. [20] 王良绪,禹涛.自体外周血干细胞的动员和采集及其移植效果的研究[J].中华内科杂志,1995,34(10):659-662. [21] Maiolino A, Hungria VT, Garnica M, et al. Thalidomide plus dexamethasone as a maintenance therapy after autologous hematopoietic stem cell transplantation improves progression-free survival in multiple myeloma. Am J Hematol. 2012;87(10):948-952. [22] Liles WC, Huang JE, Llewellyn C, et al. A comparative trial of granulocyte-colony-stimulating factor and dexamethasone, separately and in combination, for the mobilization of neutrophils in the peripheral blood of normal volunteers. Transfusion. 1997;37(2):182-187. [23] Liles WC, Rodger E, Dale DC. Combined administration of G-CSF and dexamethasone for the mobilization of granulocytes in normal donors: optimization of dosing. Transfusion. 2000;40(6): 642-644. [24] Heuft HG, Goudeva L, Sel S, et al. Equivalent mobilization and collection of granulocytes for transfusion after administration of glycosylated G-CSF (3 microg/kg) plus dexamethasone versus glycosylated G-CSF (12 microg/kg) alone. Transfusion. 2002;42(7):928-934. [25] Kikukawa Y, Yuki H, Hirata S, et al. Combined use of bortezomib, cyclophosphamide, and dexamethasone induces favorable hematological and organ responses in Japanese patients with amyloid light-chain amyloidosis: a single-institution retrospective study. Int J Hematol. 2015;101(2):133-139. [26] Cavo M, Pantani L, Petrucci MT, et al. Bortezomib-thalidomide-dexamethasone is superior to thalidomide-dexamethasone as consolidation therapy after autologous hematopoietic stem cell transplantation in patients with newly diagnosed multiple myeloma. Blood. 2012;120(1):9-19. [27] Kessler K, Goudeva L, Heuft HG. Lenograstim with or without dexamethasone for neutrophil mobilization in healthy donors: short-term kinetics of white blood cells and effects of granulocyte apheresis. J Clin Apher. 2011;26(6):338-346. [28] Kucia M, Jankowski K, Reca R, et al. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol. 2004;35(3):233-245. [29] Ma Q, Jones D, Borghesani PR, et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A. 1998; 95(16):9448-9453. [30] Slater S.Plerixafor. J Adv Pract Oncol. 2012;3(1): 49-54. [31] Dar A, Schajnovitz A, Lapid K, et al. Rapid mobilization of hematopoietic progenitors by AMD3100 and catecholamines is mediated by CXCR4-dependent SDF-1 release from bone marrow stromal cells. Leukemia. 2011;25(8):1286-1296. [32] Cashen AF. Plerixafor hydrochloride: a novel agent for the mobilization of peripheral blood stem cells. Drugs Today (Barc). 2009;45(7):497-505. [33] Krause DS, Ito T, Fackler MJ, et al. Characterization of murine CD34, a marker for hematopoietic progenitor and stem cells. Blood. 1994;84(3):691-701. [34] Lataillade JJ, Clay D, Dupuy C, et al. Chemokine SDF-1 enhances circulating CD34(+) cell proliferation in synergy with cytokines: possible role in progenitor survival. Blood. 2000;95(3):756-768. [35] Villa CH, Shore T, Van Besien K, et al. Addition of plerixafor to mobilization regimens in autologous peripheral blood stem cell transplants does not affect the correlation of preharvest hematopoietic precursor cell enumeration with first-harvest CD34+ stem cell yield. Biol Blood Marrow Transplant. 2012;18(12): 1867-1875. [36] Maziarz RT, Nademanee AP, Micallef IN, et al. Plerixafor plus granulocyte colony-stimulating factor improves the mobilization of hematopoietic stem cells in patients with non-Hodgkin lymphoma and low circulating peripheral blood CD34+ cells. Biol Blood Marrow Transplant. 2013;19(4):670-675. [37] 李艳萍,彭世义.穴位注射地塞米松治疗Ⅳ度白细胞减少疗效观察[J].现代医药卫生, 2014, 30(18):2796-2797. [38] 胡晶,吴宏.当归多糖对小鼠外周血造血干细胞动员作用的研究[J].中草药, 2007, 37(12): 1835-1838. [39] Papoff P, Christensen RD, Harcum J, et al. In vitro effect of dexamethasone phosphate on hematopoietic progenitor cells in preterm infants. Arch Dis Child Fetal Neonatal Ed. 1998;78(1):F67-69. [40] Golde DW, Bersch N, Quan SG, et al. Inhibition of murine granulopoiesis in vitro by dexamethasone. Am J Hematol. 1976;1(4):369-373. [41] Winkler IG, Pettit AR, Raggatt LJ, et al. Hematopoietic stem cell mobilizing agents G-CSF, cyclophosphamide or AMD3100 have distinct mechanisms of action on bone marrow HSC niches and bone formation. Leukemia. 2012;26(7):1594-1601. [42] Caulfield J, Fernandez M, Snetkov V, et al. CXCR4 expression on monocytes is up-regulated by dexamethasone and is modulated by autologous CD3+ T cells. Immunology. 2002;105(2):155-162. [43] Ghosh MC, Baatar D, Collins G, et al. Dexamethasone augments CXCR4-mediated signaling in resting human T cells via the activation of the Src kinase Lck. Blood. 2009;113(3):575-584. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [3] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [4] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [5] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [6] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [7] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [8] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [9] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [10] | Xing Hao, Zhang Yonghong, Wang Dong. Advantages and disadvantages of repairing large-segment bone defect [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 426-430. |
| [11] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| [12] | Wang Hao, Chen Mingxue, Li Junkang, Luo Xujiang, Peng Liqing, Li Huo, Huang Bo, Tian Guangzhao, Liu Shuyun, Sui Xiang, Huang Jingxiang, Guo Quanyi, Lu Xiaobo. Decellularized porcine skin matrix for tissue-engineered meniscus scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3473-3478. |
| [13] | Mo Jianling, He Shaoru, Feng Bowen, Jian Minqiao, Zhang Xiaohui, Liu Caisheng, Liang Yijing, Liu Yumei, Chen Liang, Zhou Haiyu, Liu Yanhui. Forming prevascularized cell sheets and the expression of angiogenesis-related factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3479-3486. |
| [14] | Liu Chang, Li Datong, Liu Yuan, Kong Lingbo, Guo Rui, Yang Lixue, Hao Dingjun, He Baorong. Poor efficacy after vertebral augmentation surgery of acute symptomatic thoracolumbar osteoporotic compression fracture: relationship with bone cement, bone mineral density, and adjacent fractures [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3510-3516. |
| [15] | Liu Liyong, Zhou Lei. Research and development status and development trend of hydrogel in tissue engineering based on patent information [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3527-3533. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||