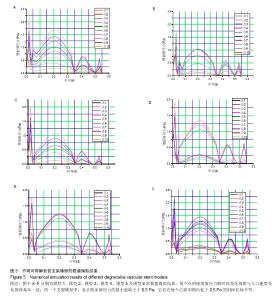
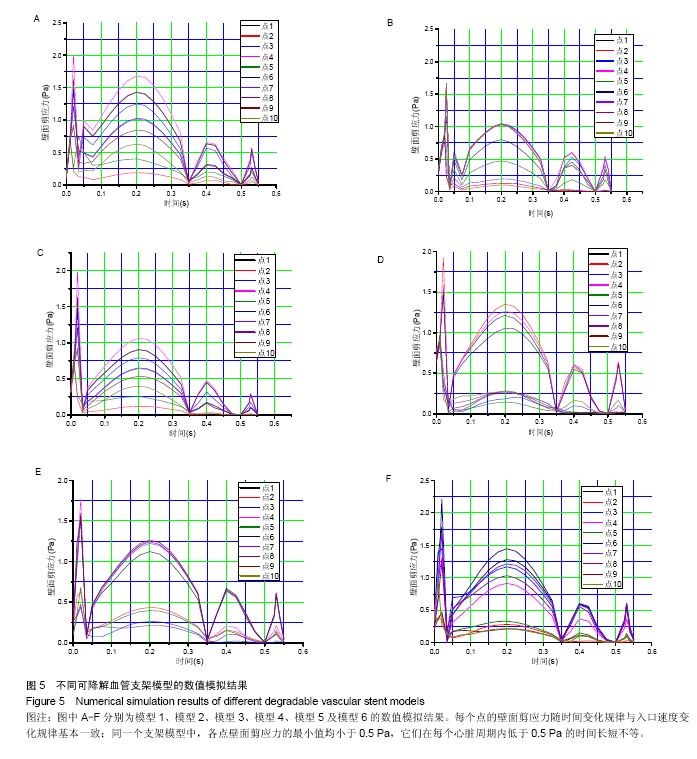
| [1]Health Quality Ontario.Stenting for peripheral artery disease of the lower extremities: an evidence-based analysis.Ont Health Technol Assess Ser.2010;10(18):1-88.[2]Palmerini T,Biondi-Zoccai G,Della Riva D,et al.Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis.Lancet(London, England). 2012;379:1393-1402.[3]Cortese B,Bertoletti A,De Matteis S,et al.Drug-eluting stents perform better than bare metal stents in small coronary vessels: a meta-analysis of randomised and observational clinical studies with mid-term follow up.Int J Cardiol. 2012; 161(2):73-82. [4]Grube E,Chevalier B,Smits P,et al.The SPIRIT V study: a clinical evaluation of the XIENCE V everolimus-eluting coronary stent system in the treatment of patients with de novo coronary artery lesions.JACC Cardiovasc Interv.2011;4: 168-175.[5]Iqbal J,Onuma Y,Ormiston J,et al.Bioresorbable scaffolds: rationale, current status, challenges, and future.Eur Heart J.2014;35:765-776.[6]Lesiak M,Araszkiewicz A."Leaving nothing behind": is the bioresorbable vascular scaffold a new hope for patients with coronary artery disease?Postepy Kardiol Interwencyjnej. 2014;10(4):283-288. [7]Hutmacher DW.Scaffolds in tissue engineering bone and cartilage.Biomaterials.2000;21:2529-2543.[8]Agrawal CM,Haas KF,Leopold DA,et al.Evaluation of poly(L-lactic acid) as a material for intravascular polymeric stents.Biomaterials.1992;13:176-182.[9]Patel N,Banning AP.Bioabsorbable scaffolds for the treatment of obstructive coronary artery disease: the next revolution in coronary intervention? Heart(British Cardiac Society).2013; 99:1236-1243.[10]Kassimis G,Spiliopoulos S,Katsanos K,et al.Bioresorbable scaffolds in peripheral arterial disease. Expert Rev Cardiovasc Ther.2014;12:443-450.[11]Onuma Y,Serruys PW.Bioresorbable scaffold: the advent of a new era in percutaneous coronary and peripheral revascularization? Circulation.2011;123:779-797.[12]Nishio S,Kosuga K,Igaki K,et al.Long-Term (>10 Years) clinical outcomes of first-in-human biodegradable poly-l-lactic acid coronary stents: Igaki-Tamai stents.Circulation. 2012; 125:2343-2353.[13]Garg S,Serruys PW.Coronary stents: looking forward.J Am Coll Cardiol.2010;56:S43-78.[14]Tamai H,Igaki K,Kyo E,et al.Initial and 6-month results of biodegradable poly-l-lactic acid coronary stents in humans. Circulation.2000;102:399-404.[15]Ormiston JA,Serruys PW,Regar E,et al.A bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions (ABSORB): a prospective open-label trial. Lancet(London, England). 2008;371:899-907.[16]Okamura T,Garg S,Gutierrez-Chico JL,et al.In vivo evaluation of stent strut distribution patterns in the bioabsorbable everolimus-eluting device: an OCT ad hoc analysis of the revision 1.0 and revision 1.1 stent design in the ABSORB clinical trial.EuroIntervention.2010;5(8):932-938.[17]Koskinas KC,Chatzizisis YS,Antoniadis AP,et al.Role of endothelial shear stress in stent restenosis and thrombosis: pathophysiologic mechanisms and implications for clinical translation. J Am Coll Cardiol.2012;59(15):1337-1349.[18]Friedman MH,Bargeron CB,Deters OJ,et al.Correlation between wall shear and intimal thickness at a coronary artery branch.Atherosclerosis.1987;68:27-33.[19]Chatzizisis YS,Coskun AU,Jonas M,et al.Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol.2007;49:2379-2393.[20]Stone PH,Coskun AU,Kinlay S,et al.Regions of low endothelial shear stress are the sites where coronary plaque progresses and vascular remodelling occurs in humans: an in vivo serial study.Eur Heart J.2007;28:705-710.[21]Samady H,Eshtehardi P,McDaniel MC,et al.Coronary artery wall shear stress is associated with progression and transformation of atherosclerotic plaque and arterial remodeling in patients with coronary artery disease. Circulation. 2011;124:779-788.[22]Stone PH,Saito S,Takahashi S,et al.Prediction of progression of coronary artery disease and clinical outcomes using vascular profiling of endothelial shear stress and arterial plaque characteristics: the PREDICTION Study.Circulation. 2012;126:172-181.[23]Jenei C,Balogh E,Szabo GT,et al.Wall shear stress in the development of in-stent restenosis revisited. A critical review of clinical data on shear stress after intracoronary stent implantation. Cardiol J.2016;23:365-373.[24]Berry JL,Santamarina A,Moore JE Jr,et al.Experimental and computational flow evaluation of coronary stents.Ann Biomed Eng.2000;28:386-398.[25]Morlacchi S,Keller B,Arcangeli P,et al.Hemodynamics and in-stent restenosis: micro-CT images, histology, and computer simulations.Ann Biomed Eng.2011;39:26152626.[26]LaDisa JF Jr,Olson LE,Guler I,et al.Circumferential vascular deformation after stent implantation alters wall shear stress evaluated with time-dependent 3D computational fluid dynamics models.J Appl Physiol(1985).2005;98(3):947-957. [27]Edelman ER,Rogers C.Pathobiologic responses to stenting. Am J Cardiol.1998;81(7A):4E-6E.[28]Chiu JJ,Chien S.Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91(1):327-387.[29]Van der Heiden K,Gijsen FJ,Narracott A,et al.The effects of stenting on shear stress: relevance to endothelial injury and repair.Cardiovasc Res.2013;99(2):269-275. [30]Economou F,Katranas S,Giannoglou G,et al.Impact of stent implantation on endothelial shear stress. Herz.2016.[Epub ahead of print][31]Moore J Jr,Berry JL.Fluid and solid mechanical implications of vascular stenting.Ann Biomed Eng. 2002;30(4):498-508.[32]顾兴中,程洁,李俐军,等.血管支架耦合系统血流动力学数值模拟与实验研究[J].东南大学学报(自然科学版), 2012,43(6): 1089-1093.[33]欧阳墉.血液流变学及其在支架置入术后的变化[J].介入放射学杂志,2002,11(5):382-384.[34]Pakala R,Watanabe T,Benedict CR.Induction of endothelial cell proliferation by angiogenic factors released by activated monocytes.Cardiovasc Radiat Med.2002;3(2):95-101.[35]Cunningham KS,Gotlieb AI.The role of shear stress in the pathogenesis of atherosclerosis.Lab Invest.2005;85(1):9-23.[36]孙安强,邓小燕.冠状动脉支架血流动力学中的几个问题[J].生物物理学报,2011,27(8):669-675.[37]Shishido K,Antoniadis AP,Takahashi S,et al.Effects of Low Endothelial Shear Stress After Stent Implantation on Subsequent Neointimal Hyperplasia and Clinical Outcomes in Humans.J Am Heart Assoc. 2016;5(9).pii:e002949.doi:10.1161/JAHA.115.002949.[38]Frank AO,Walsh PW,Moore JE Jr.Computational fluid dynamics and stent design.Artif Organs. 2002;26(7):614-621.[39]Carter AJ,Scott D,Rahdert D,et al.Stent design favorably influences the vascular response in normal porcine coronary arteries.J Invasive Cardiol.1999;11(3):127-134.[40]邱晓宁,费智敏,张珏,等.壁面弹性对分叉颈动脉个体化病例血流动力学影响的数值模拟[J].上海交通大学学报(医学版), 2011, 31(11):1554-1559. |