Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (34): 5437-5442.doi: 10.3969/j.issn.2095-4344.2017.34.005
Previous Articles Next Articles
A novel tissue-engineered bone scaffold carrying bone morphogenetic protein 2
- Department of Traumatic Orthopedics, Inner Mongolia People’s Hospital, Hohhot 010017, Inner Mongolia Autonomous Region, China
-
Received:2017-10-14Online:2017-12-08Published:2018-01-04 -
Contact:Sun Guan-wen, Department of Traumatic Orthopedics, Inner Mongolia People’s Hospital, Hohhot 010017, Inner Mongolia Autonomous Region, China -
About author:Sun Guan-wen, M.D., Chief physician, Department of Traumatic Orthopedics, Inner Mongolia People’s Hospital, Hohhot 010017, Inner Mongolia Autonomous Region, China -
Supported by:the Natural Science Foundation of Inner Mongolia Autonomous Region, No. 2015MS0383
CLC Number:
Cite this article
Sun Guan-wen, Wang Jian, Shi Bin, Jia Peng.
share this article
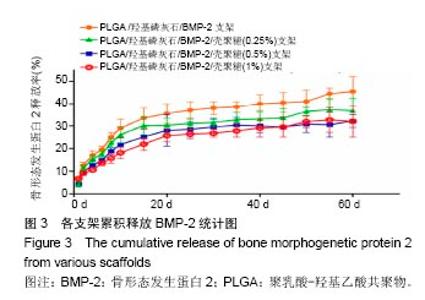
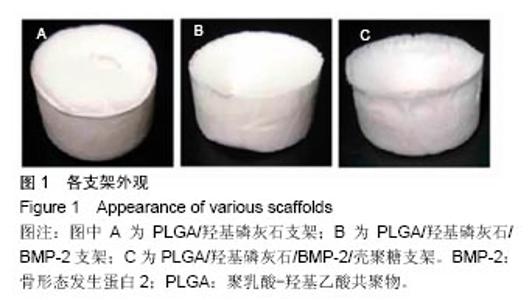
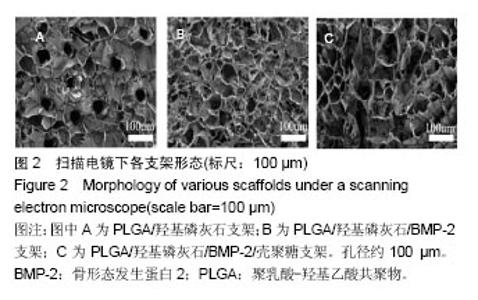
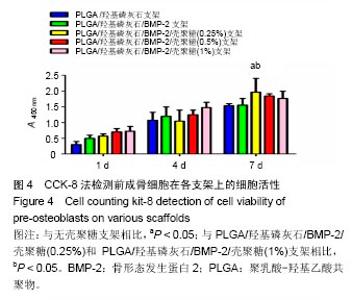
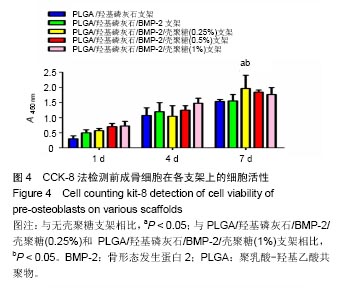
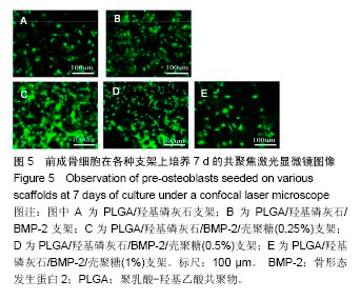
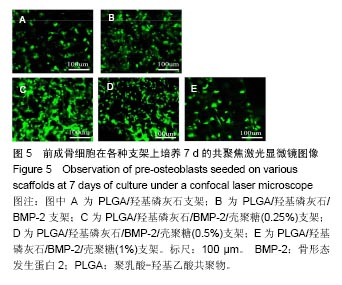
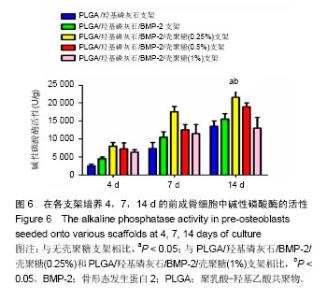
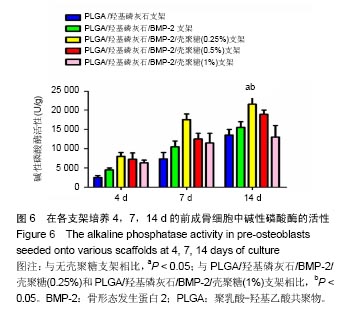
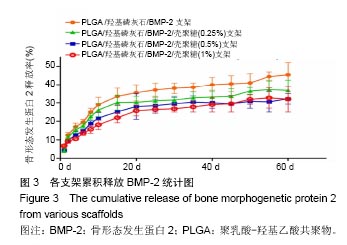
2.2 支架的亲水性 PLGA/羟基磷灰石支架吸水率只有(35.32±0.54)%,PLGA /羟基磷灰石/ BMP-2支架吸水率增加到(35.78±1.07)%。随着壳聚糖含量的增加,PLGA/羟基磷灰石/BMP-2/壳聚糖支架的吸水率呈增加趋势,分别为(37.64±0.96)%,(39.15±1.23)%,(40.28±0.59)%。提示壳聚糖提高了复合材料PLGA/羟基磷灰石/BMP-2支架的亲水性。 2.3 各支架BMP-2的释放 使用ELISA试剂盒,测定60 d内支架释放BMP-2(图3)。加载BMP-2的支架在第1天呈爆发释放,随之呈持续缓慢释放。20 d内,支架表现出突发释放和递减释放。各支架BMP-2的累计释放的顺序:PLGA/羟基磷灰石/BMP-2支架35% > PLGA/羟基磷灰石/BMP-2/壳聚糖(0.25%)支架30% > PLGA/羟基磷灰石/BMP-2/壳聚糖(0.5%)支架28% > PLGA/羟基磷灰石/BMP-2/壳聚糖(1%)支架27%。在第20-24天,各支架释放BMP-2相同。但是,BMP-2累计从PLGA/羟基磷灰石/BMP-2/壳聚糖(1%)支架的释放接近PLGA/羟基磷灰石/BMP-2/壳聚糖(0.5%)支架。在第60天时,PLGA/羟基磷灰石/BMP-2、PLGA/羟基磷灰石/BMP-2/壳聚糖(0.25%)、PLGA/羟基磷灰石/ BMP-2/壳聚糖(0.5%)、PLGA/羟基磷灰石/BMP-2/壳聚糖(1%)支架BMP-2累积释放分别为44%、34%、27%和26%。这可能是支架表面附着壳聚糖抑制BMP-2释放。"
| [1]Reichert JC, Epari DR, Wullschleger ME, et al. Establishment of a preclinical ovine model for tibial segmental bone defect repair by applying bone tissue engineering strategies. Tissue Eng Part B Rev. 2010;16(1):93-104.[2]Wang C, Wang Z, Li A, et al. Repair of segmental bone-defect of goat's tibia using a dynamic perfusion culture tissue engineering bone. J Biomed Mater Res A. 2010;92(3): 1145-1153.[3]李建军,赵群,王欢,等.基因修饰的组织工程骨联合带血管蒂骨膜移植修复长段骨缺损的研究[J].中华整形外科杂志, 2007,23(6): 502-506.[4]Ami R. Amini, Cato T. et al. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng.2012; 40(5): 363–408.[5]Delabarde C, Plummer CJ, Bourban PE, et al. Biodegradable polylactide/hydroxyapatite nanocomposite foam scaffolds for bone tissue engineering applications. J Mater Sci Mater Med. 2012;23(6):1371-1385.[6]Tripathi A, Saravanan S, Pattnaik S, et al. Bio-composite scaffolds containing chitosan/nano-hydroxyapatite/ nano-copper-zinc for bone tissue engineering. Int J Biol Macromol. 2012;50(1):294-299.[7]Brun F, Turco G, Accardo A, et al. Automated quantitative characterization of alginate/hydroxyapatite bone tissue engineering scaffolds by means of micro-CT image analysis. J Mater Sci Mater Med. 2011;22(12):2617-2629.[8]Yu NY, Schindeler A, Little DG, et al. Biodegradable poly(alpha-hydroxy acid) polymer scaffolds for bone tissue engineering. J Biomed Mater Res B Appl Biomater. 2010; 93(1):285-295.[9]Kretlow JD, Mikos AG. Review: mineralization of synthetic polymer scaffolds for bone tissue engineering. Tissue Eng. 2007;13(5):927-938.[10]Jansen EJ, Sladek RE, Bahar H, et al. Hydrophobicity as a design criterion for polymer scaffolds in bone tissue engineering. Biomaterials. 2005;26(21):4423-4431.[11]Wei G, Ma PX. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials. 2004;25(19):4749-4757.[12]Xiong JY, Li YC, Wang XJ, et al. Titanium-nickel shape memory alloy foams for bone tissue engineering. J Mech Behav Biomed Mater. 2008;1(3):269-273.[13]Warnke PH, Douglas T, Wollny P, et al. Rapid prototyping: porous titanium alloy scaffolds produced by selective laser melting for bone tissue engineering. Tissue Eng Part C Methods. 2009;15(2):115-124.[14]Ding F, Wu J, Yang Y, et al. Use of tissue-engineered nerve grafts consisting of a chitosan/poly(lactic-co-glycolic acid)-based scaffold included with bone marrow mesenchymal cells for bridging 50-mm dog sciatic nerve gaps. Tissue Eng Part A. 2010;16(12):3779-3790.[15]郝增涛,冯卫,郝廷,等.BMSCs来源成骨细胞和内皮细胞复合壳聚糖-羟基磷灰石多孔支架构建血管化组织工程骨研究[J].中国修复重建外科杂志,2012,26(4):489-494.[16]王新,刘玲蓉,张其清.纳米羟基磷灰石-壳聚糖骨组织工程支架的研究[J].中国修复重建外科杂志,2007,21(2):120-124.[17]Kim HW, Noh YJ, Koh YH, et al. Enhanced performance of fluorine substituted hydroxyapatite composites for hard tissue engineering. J Mater Sci Mater Med. 2003;14(10):899-904.[18]Zhang R, Ma PX. Poly(alpha-hydroxyl acids)/hydroxyapatite porous composites for bone-tissue engineering. I. Preparation and morphology. J Biomed Mater Res.1999;44(4):446-455.[19]Link DP, van den Dolder J, Jurgens WJ, et al. Mechanical evaluation of implanted calcium phosphate cement incorporated with PLGA microparticles. Biomaterials. 2006; 27(28):4941-4947.[20]Zhang Q, Mochalin VN, Neitzel I, et al. Mechanical properties and biomineralization of multifunctional nanodiamond-PLLA composites for bone tissue engineering. Biomaterials. 2012; 33(20):5067-5075.[21]Sultana N, Wang M. PHBV/PLLA-based composite scaffolds fabricated using an emulsion freezing/freeze-drying technique for bone tissue engineering: surface modification and in vitro biological evaluation. Biofabrication. 2012;4(1):015003.[22]Ngiam M, Liao S, Patil AJ, et al. The fabrication of nano-hydroxyapatite on PLGA and PLGA/collagen nanofibrous composite scaffolds and their effects in osteoblastic behavior for bone tissue engineering. Bone. 2009;45(1):4-16.[23]Hou J, Wang J, Cao L, et al. Segmental bone regeneration using rhBMP-2-loaded collagen/chitosan microspheres composite scaffold in a rabbit model. Biomed Mater. 2012; 7(3):035002.[24]Oliveira JM, Rodrigues MT, Silva SS, et al. Novel hydroxyapatite/chitosan bilayered scaffold for osteochondral tissue-engineering applications: Scaffold design and its performance when seeded with goat bone marrow stromal cells. Biomaterials. 2006;27(36):6123-6137.[25]Park KH, Kim H, Moon S, et al. Bone morphogenic protein-2 (BMP-2) loaded nanoparticles mixed with human mesenchymal stem cell in fibrin hydrogel for bone tissue engineering. J Biosci Bioeng. 2009;108(6):530-537.[26]Sohier J, Daculsi G, Sourice S, et al. Porous beta tricalcium phosphate scaffolds used as a BMP-2 delivery system for bone tissue engineering. J Biomed Mater Res A. 2010;92(3): 1105-1114.[27]Kempen DH, Lu L, Hefferan TE, et al. Retention of in vitro and in vivo BMP-2 bioactivities in sustained delivery vehicles for bone tissue engineering. Biomaterials. 2008;29(22):3245-3252.[28]袁泉.载骨形态发生蛋白2活性肽的纳米仿生骨基质材料的实验研究[D].华中科技大学,2017.[29]Waghela BN, Sharma A, Dhumale S, et al. Curcumin conjugated with PLGA potentiates sustainability, anti-proliferative activity and apoptosis in human colon carcinoma cells. PLoS One.2015;10(2):e0117526.[30]刘莉,常红,黄国伟,等.体外培养大鼠成骨细胞实验模型的建立[J].天津医科大学学报,2004,10(1):39-42[31]许瑾,吴晶晶,王晓冬,王秀梅,韩倩倩.羟基磷灰石复合骨组织工程支架的研究进展[J].生物骨科材料与临床研究, 2016,13(2): 63-66.[32]Vinn SR, Hu Y, Sfeir C, et al. Gene therapy approaches for modulating bone regeneration. AdVDtug DeliV Rev. 2000;42: 12l-138.[33]Wang EA, Rosen V, Cordes P, et al. Purification and characterization of other distinct bone-inducing factors. Proc Nat Acad Sci USA. 2015;85:9484-9488. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [3] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [4] | Yang Feng, Zhao Qian, Zhang Shixuan, Zhao Tienan, Feng Bo. Effectiveness and safety of rapamycin combined with CD133 antibody stent in preventing vascular restenosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 579-584. |
| [5] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [6] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [7] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [8] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [9] | Li Hui, Chen Lianglong. Application and characteristics of bone graft materials in the treatment of spinal tuberculosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 626-630. |
| [10] | Gao Cangjian, Yang Zhen, Liu Shuyun, Li Hao, Fu Liwei, Zhao Tianyuan, Chen Wei, Liao Zhiyao, Li Pinxue, Sui Xiang, Guo Quanyi. Electrospinning for rotator cuff repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 637-642. |
| [11] | Le Guoping, Zhang Ming, Xi Licheng, Luo Hanwen. Preparation and in vitro evaluation of vancomycin hydrochloride@polylactic acid-glycolic acid copolymer-chitosan-hyaluronic acid composite sustained-release microspheres [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 528-534. |
| [12] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [13] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [14] | Guan Jian, Jia Yanfei, Zhang Baoxin , Zhao Guozhong. Application of 4D bioprinting in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 446-455. |
| [15] | Liu Jiali, Suo Hairui, Yang Han, Wang Ling, Xu Mingen. Influence of lay-down angles on mechanical properties of three-dimensional printed polycaprolactone scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2612-2617. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||