Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (32): 5203-5206.doi: 10.3969/j.issn.2095-4344.2017.32.020
Previous Articles Next Articles
Role of 11beta-hydroxysteroid dehydrogenase in bone metabolism
Han Jun1, Sun Wei2, Gao Fu-qiang2
- (1China-Japan Friendship Clinical Medical School, Peking University, Beijing 100029, China; 2Department of Bone and Joint Surgery, China-Japan Friendship Hospital, Beijing 100029, China)
-
Received:2017-08-07Online:2017-11-18Published:2017-11-15 -
Contact:Gao Fu-qiang, Attending physician, Department of Bone and Joint Surgery, China-Japan Friendship Hospital, Beijing 100029, China -
About author:Han Jun, Studying for master’s degree, China-Japan Friendship Clinical Medical School, Peking University, Beijing 100029, China Sun Wei, Chief physician, Professor, Doctoral supervisor, Master’s supervisor, Department of Bone and Joint Surgery, China-Japan Friendship Hospital, Beijing 100029, China Han Jun and Sun Wei contributed equally to this work. -
Supported by:the Natural Science Foundation of Beijing, No. 7174346; the General Project of National Natural Science Foundation of China, No. 81372013 and 81672336; the Project of China-Japan Friendship Hospital, No. 2014-4-QN-29; the Science and Technology Plan for Young Talents of China-Japan Friendship Hospital, No. 2014-QNYC-A-06
CLC Number:
Cite this article
Han Jun1, Sun Wei2, Gao Fu-qiang2. Role of 11beta-hydroxysteroid dehydrogenase in bone metabolism[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(32): 5203-5206.
share this article
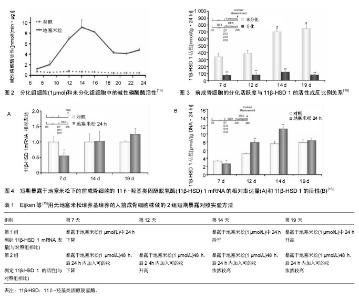
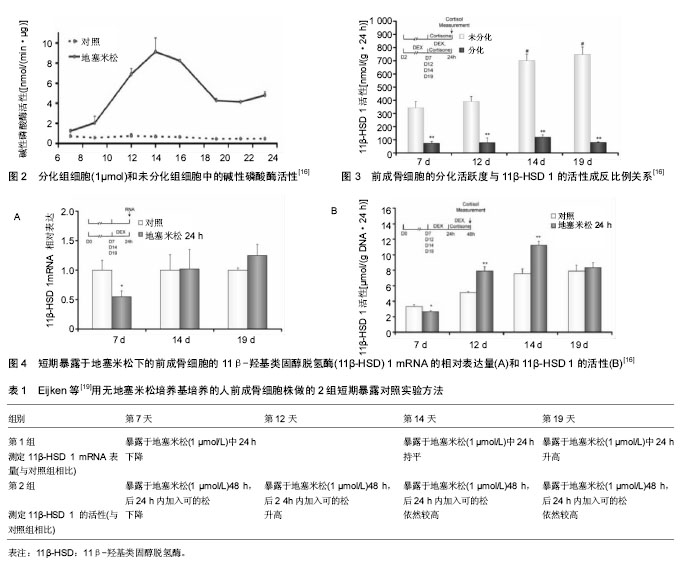
2 结果 Results 2.1 11β-HSD的基本结构及对糖皮质激素的调节 11β-HSD属于短肽链乙醇氧化还原酶家族,以N-末端结合于特定细胞的内质网上,另外接触反应区还存在一个佪文结构,类似于于糖皮质激素的反应位点。该酶分为两型,即11β-HSD1和11β-HSD2。11β-HSD1有292个氨基酸,相对分子质量为34×103。11β-HSD1在多肽链上有两个糖基化位点,糖基化抑制剂可削弱11β-HSD1的脱氢酶功能,但不影响还原酶功能[7]。人类11β-HSD2有405个氨基酸,相对分子质量约为40×103。11β-HSD2在人体中发挥脱氢酶的作用,目前未发现具有还原酶作用的11β-HSD2。11β-HSD2结构中也有一两个潜在的糖基化位点,由于其周围存在脯氨酸,因此被糖基化的可能性很小[8]。 由于11β-HSD 1和11β-HSD 2的催化功能不同,两者在体内的分布具有鲜明的特点。11β-HSD 1主要分布于成骨细胞和破骨细胞中[9],11β-HSD 2则主要分布于正常胎儿骨骼和骨肉瘤细胞中[9-10]。11β-HSD 1具有氧化和还原的双重催化作用, 在骨细胞完整情况下主要发挥还原酶作用,可以将细胞内的无活性的皮质酮还原成有活性的皮质醇,所以11β-HSD 1又被称为“糖皮质激素效应的扩大器”。11β-HSD 2为专一的氧化酶,它通过氧化作用使糖皮质激素失活,从而降低细胞局部的糖皮质激素浓度。11β-HSD 1和11β-HSD 2两者共同作用,使细胞局部的糖皮质激素浓度达到生理平衡状态,从而维持糖皮质激素对正常骨代谢的生理作用。 2.2 11β-HSD 调控骨代谢的生理机制 因为11β-HSD在成骨细胞和破骨细胞中都有分布,通过对糖皮质激素活性的调节,可以对骨代谢的整个过程进行调节,影响骨骼的生长发育,因而11β-HSD对于维持骨代谢的正常运转非常的重要[11]。11β-HSD 1的活性和11β-HSD 1 mRNA表达量与成骨标志物的水平有密切关系[12]。以往的研究发现,个体对糖皮质激素敏感度(即:成骨标志物水平的下降程度)的差异化,与循环血液的糖皮质激素浓度及骨细胞表面糖皮质激素受体的分布密度都无明显关联[13-15]。Cooper等[12]研究发现,11β-HSD 1的活性与个体对糖皮质激素的敏感度呈正比例关系,Cooper等[12]对20名健康的志愿者首先进行基础11β-HSD 1活性的测定,然后每人每次口服5 mg泼尼松龙,2次/d,持续 7 d。发现志愿者血清中的成骨标志物水平明显下降,而破骨标志物水平变化不大,成骨标志物的下降趋势与服用激素前11β-HSD 1的活性趋势呈正比例关系,但与11β-HSD 2的活性趋势没有比例关系。11β-HSD1在破骨细胞中亦有分布,Kaji等[16]认为,糖皮质激素对破骨细胞的分化有重要的促进作用,根据Cooper等[17]的研究发现,志愿者服用11β-HSD非特异性抑制剂甘珀酸后,骨吸收标志物下降明显,而骨形成标志物无明显改变。Park等[18]应用特异性11β-HSD1抑制剂KR-67500之后,实验小鼠体内也出现了与上述实验结果一样的变化。11β-HSD1作为一种还原酶,对于提高破骨细胞内的糖皮质激素水平有重要作用,而甘珀酸抑制了11β-HSD1的活动后,将会减弱对糖皮质激素的活化作用,从而导致骨吸收标志下降。 成骨细胞的11β-HSD 1活性与其分化活跃度密切相关。Eijken等[19]研究发现其两者呈反比例关系,他们应用人前成骨细胞株(SV-HFO)进行体外实验,实验分为加有1 μmol地塞米松培养基的实验组(分化组)和不含地塞米松培养基的对照组(非分化组),培养23 d后测定每组碱性磷酸酶(成骨细胞的分化活跃度)的含量,发现实验组的碱性磷酸酶含量更高,所以地塞米松对前成骨细胞具有促分化作用(见图2)。此后,分别测定2组的11β-HSD 1分化程度(在培养7,12,14,19 d时,各添加1 μmol/L的可的松,以测定每组的11β-HSD 1的活性),发现不含地塞米松组的11β-HSD 1的活性更高,所以前成骨细胞的分化活跃度与11β-HSD 1的活性成反比例关系(见图3),11β-HSD 1可以反馈调节前成骨细胞局部的糖皮质激素的浓度,以此达到糖皮质激素的浓度平衡,从而维持正常的骨代谢[19]。为了进一步验证这一结论,Eijken等[19]又将SV-HFO分别在不同浓度梯度的地塞米松条件下培养19 d,发现碱性磷酸酶水平呈激素浓度依赖性升高,同时,11β-HSD 1的活性呈激素浓度依赖性降低。然而,有些研究发现,11β-HSD 1的活性与糖皮质激素的浓度呈正比[20-21],但Eijken等[19]学者认为,11β-HSD 1的活性与糖皮质激素的浓度之间的关系分两种情况,第1种情况是SV-HFO短期暴露于地塞米松的条件下,地塞米松可以直接促进11β-HSD 1 mRNA的表达。第2种情况则是SV-HFO长期暴露于地塞米松的条件下,地塞米松通过促进SV-HFO的分化,从而间接抑制了11β-HSD 1 mRNA的表达。Eijken等用无地塞米松培养基培养的SV-HFO做了两组短期暴露对照实验,第1组对照试验是测定11β-HSD 1 mRNA的相对表达量组(见图4A),第2组对照试验是测定11β-HSD 1 的活性组(见图4B),见表1。 结果显示,培养12 d之后的SV-HFO,短期的地塞米松暴露可以促进11β-HSD 1的表达。而对于培养第7天时,短期暴露于地塞米松的SV-HFO,其11β-HSD 1 mRNA表达量下降是因为SV-HFO在7d内对于糖皮质激素诱导分化的敏感性很高,从而高分化活跃度抑制了11β-HSD 1 mRNA表达量,12 d之后的11β-HSD 1 mRNA表达量升高则是糖皮质激素的直接刺激导致。 11β-HSD 2的活性影响成骨细胞的发育及成熟。研究表明组织局部活性糖皮质激素浓度在间叶细胞分化为成熟的成骨细胞中起到了关键作用[22-23]。但是,从Col2.3-11β-HSD2转基因小鼠实验(阻断糖皮质激素信号通路)得到的证据显示,糖皮质激素不是直接作用于间叶细胞,而是以成熟成骨细胞作为中间媒介[24],间接作用于间叶细胞。比如,对取自Col2.3-11β-HSD2转基因小鼠的颅骨组织进行培养,与具有完好糖皮质激素通路的野生型小鼠相比较,表现为间叶细胞倾向于向脂肪细胞分化,而分化为成骨细胞的数量减少。在异常表型的 "
| [1] Levinger I, Seeman E, Jerums G, et al. Glucose-loading reduces bone remodeling in women and osteoblast function in vitro. Physiol Rep. 2016;4(3). pii: e12700. [2] Cheng SL, Zhang SF, Avioli LV. Expression of bone matrix proteins during dexamethasone-induced mineralization of human bone marrow stromal cells. J Cell Biochem. 1996; 61(2):182-193.[3] Iba K, Chiba H, Sawada N,et al. Glucocorticoids induce mineralization coupled with bone protein expression without influence on growth of a human osteoblastic cell line.Cell Struct Funct. 1995;20(5):319-330.[4] Weinstein RS, Jilka RL, Parfitt AM,et al.Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102(2): 274-282. [5] Hirayama T, Sabokbar A, Athanasou NA. Effect of corticosteroids on human osteoclast formation and activity. The Journal of endocrinology. 2002;175(1):155-63.[6] Stewart PM, Krozowski ZS. 11 beta-Hydroxysteroid dehydrogenase. Vitam Horm. 1999;57:249-324. [7] Yang K, Smith CL, Dales D,et al. Cloning of an ovine 11 beta-hydroxysteroid dehydrogenase complementary deoxyribonucleic acid: tissue and temporal distribution of its messenger ribonucleic acid during fetal and neonatal development. Endocrinology. 1992;131(5):2120-6.[8] Brown RW, Chapman KE, Kotelevtsev Y, et al.Cloning and production of antisera to human placental 11β-hydroxysteroid dehydrogenase type 2. Biochem J. 1996;313(Pt 3): 1007-1017.[9] Bland R, Worker CA, Noble BS, et al. Characterization of 11β-hydroxysteroid dehydrogenase activity and corticosteroid receptor expression in human osteosarcoma cell lines. J Endocrinol. 1999;161(3):455-464.[10] Condon J, Gosden C, Gardener D, et al.Expression of Type 2 11 -Hydroxysteroid Dehydrogenase and Corticosteroid Hormone Receptors in Early Human Fetal Life.J Clin Endocrinol Metab. 1998;83(12):4490-4497.[11] 赵佳,魏波,刘军,等. 皮质酮对大鼠股骨头微环境11β-HSD1表达及骨重建影响的初步实验研究[J]. 重庆医学. 2016,45(3): 336-338.[12] Cooper MS, Blumsohn A, Goddard PE,et al. 11beta-hydroxysteroid dehydrogenase type 1 activity predicts the effects of glucocorticoids on bone. J Clin Endocrinol Metab. 2003;88(8):3874-3877.[13] Chiodini I, Carnevale V, Torlontano M, et al. Alterations of Bone Turnover and Bone Mass at Different Skeletal Sites due to Pure Glucocorticoid Excess: Studyin Eumenorrheic Patients with Cushing’s Syndrome. J Clin Endocrinol Metab. 1998;83(6):1863-1867.[14] Dennison E, Hindmarsh P, Fall C, et al. Profiles of Endogenous Circulating Cortisol and Bone Mineral Density in Healthy Elderly Men*. J Clin Endocrinol Metab.1999;84: 3058-3063.[15] Huizenga NA, Koper JW, De Lange P,et al. A Polymorphism in the Glucocorticoid Receptor Gene May Be Associated with an Increased Sensitivity to Glucocorticoids in Vivo. J Clin Endocrinol Metab. 1998;83(1):144-151.[16] Kaji H, Sugimoto T, Kanatani M, et al. Dexamethasone stimulates osteoclast-like cell formation by directly acting on hemopoietic blast cells and enhances osteoclast-like cell formation stimulated by parathyroid hormone and prostaglandin E2. J Bone Miner Res.1997;12(5):734-741.[17] Cooper MS, Walker EA, Bland R, et al. Expression and Functional Consequences of 11β-Hydroxysteroid Dehydrogenase Activity in Human Bone. Bone.2000;27: 375-381.[18] Park JS, Bae SJ, Choi SW, et al. A novel 11βHSD1 inhibitor improves diabesity and osteoblast Differentiation. J Mol Endocrinol. 2014 ;52(2):191-202.[19] Eijken M,Hewison M,Cooper MS,et al.11beta-Hydroxysteroid dehydrogenase expression and glucocorticoid synthesis are directed by a molecular switch during osteoblast differentiation. Mol Endocrinol. 2005;19(3):621-631.[20] Ramli ES, Suhaimi F, Asri SF, et al. Glycyrrhizic acid (GCA) as 11beta-hydroxysteroid dehydrogenase inhibitor exerts protective effect against glucocorticoid-induced osteoporosis. J Bone Miner Metab. 2013;31(3):262-273.[21] Cooper MS , Rabbitt EH , Goddard PE,et al.Osteoblastic 11 -Hydroxysteroid Dehydrogenase Type 1Activity Increases With Age and Glucocorticoid Exposure. J Bone Miner Res 2002;17 (6): 979-86.[22] Haynesworth SE, Goshima J, Goldberg VM,et al.Characterization of cells with osteogenic potential from human marrow. Bone.1992;13(1):81-88.[23] Herbertson A, Aubin JE.Dexamethasone alters the subpopulation make-up of rat bone marrow stromal cell cultures.J Bone Miner Res.1995;10(2):285-294.[24] Zhou H, Mak W, Kalak R,et al.Glucocorticoid-dependent Wnt signaling by mature osteoblastsis a key regulator of cranial skeletal development in mice. Development.2009;136(3):427-436.[25] Van Staa TP, Leufkens HG, Abenhaim L,et al. Use of oral corticosteroids and risk of fractures. June, 2000.J Bone Miner Res. 2005 ;20(8):1487-494.[26] Canalis E. Clinical review 83: Mechanisms of glucocorticoid action in bone: implications to glucocorticoid-induced osteoporosis. J Clin Endocrinol Metab. 1996;81(10): 3441-3447 [27] Brockstedt H, Kassem M, Eriksen EF,et al.Age- and sex-related changes in iliac cortical bone mass and remodeling. Bone.1993,14(4):681-691.[28] Clarke BL, Ebeling PR, Jones JD,et al.Changes in quantitative bone histomorphometry in aging healthy men. J Clin Endocrinol Metab. 1996;81(6):2264-2270.[29] Hardy RS, Raza K, Cooper MS. Glucocorticoid metabolism in rheumatoid arthritis. Ann N Y Acad Sci. 2014;1318:18-26. [30] Hardy R,Rabbitt EH,Filer A,et al.Local and systemic glucocorticoid metabolism in inflammatory arthritis. Ann Rheum Dis. 2008;67(9):1204-1210. [31] 徐杰,李国平,唐蔚青,等. I型11β羟基类固醇脱氢酶在肿瘤坏死因子-α诱导的胰岛素抵抗中的作用[J]. 中年老年医学杂志. 2016,35(5): 537-542.[32] Cai TQ, Wong B, Mundt SS, et al. Induction of 11beta-hydroxysteroid dehydrogenase type 1 but not -2 in human aortic smooth muscle cells by inflammatory stimuli. J Steroid Biochem Mol Biol. 2001;77(2-3):117-22.[33] Escher G, Galli I, Vishwanath BS, et al. Tumor Necrosis Factorа Interleukin1βEnhance the Cortisone/Cortisol Shuttle. J Exp Med. 1997;186(2):189-198.[34] Kaur K,Hardy R,Ahasan MM,et al.Synergistic induction of local glucocorticoid generation by inflammatory cytokines and glucocorticoids: implications for inflammation associated bone loss. Ann Rheum Dis. 2010;69(6):1185-1190.[35] Brabnikova Maresova K, Pavelka K, Stepan JJ.Acute effects of glucocorticoids on serum markers of osteoclasts, osteoblasts, and osteocytes. Calcif Tissue Int. 2013;92(4): 354-361. [36] Wang FS, Ko JY, Yeh DW, et al. Modulation of Dickkopf-1 Attenuates Glucocorticoid Induction of Osteoblast Apoptosis, Adipocytic Differentiation, and Bone Mass Loss. Endocrinology. 2008;149(4):1793-801.[37] Cooper MS, Bujalska I, Rabbitt E, et al. Modulation of 11beta-hydroxysteroid dehydrogenase isozymes by proinflammatory cytokines in osteoblasts: an autocrine switch from glucocorticoid inactivation to activation. J Bone Miner Res. 2001 ;16(6):1037-1044. [38] Kostadinova RM, Nawrocki AR, Frey FJ, et al. Tumor necrosis factor alpha and phorbol 12-myristate-13-acetate down-regulate human 11beta-hydroxysteroid dehydrogenase type 2 through p50/p50 NF-kappaB homodimers and Egr-1. FASEB J. 2005;19(6):650-652.[39] Li F, Qiao C, Li Y, et al. Blockade of 11β-hydroxysteroid dehydrogenase type 1 enzyme inhibits experimental collagenase-induced osteoarthritis. Mol Med Rep. 2015;11(3): 2071-2075.[40] Doig CL, Bashir J, Zielinska AE, et al. TNFa-mediated Hsd11b1 binding of NF-kB p65 is associated with suppression of 11b-HSD1 in muscle. J Endocrinol. 2014;220(3):389-396.[41] Nakano D, Nishiyama A. Programmed 11beta-hydroxysteroid dehydrogenase type 2 reduction: a possible cause of adult-onset disease?. J Hypertens. 2011;29(2):201-203.[42] Kerachian MA, Seguin C, Harvey EJ. Glucocorticoids in osteonecrosis of the femoral head: a new understanding of the mechanisms of action.J Steroid Biochem Mol Biol.2009; 114(3-5):121-128.[43] 李璐,辛婧,薛一,等. 11-HSD1与S100A16共同调节3T3-L1脂肪细胞的分化[J]. 中华内分泌代谢杂志,2014,30(9):779-85.[44] Wang L,Luo DK,Pan ZY.Expression of 11beta-HSD in steroid-induced avascular necrosis of the femoral head. Mol Med Rep.2013;7(5):1482-1486.[45] 李玉英, 王陈钱.地塞米松对血管内皮细胞11-HSD1基因表达的影响[J]. 解放军医学杂志,2007,32(10):1066-1068. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [3] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [4] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [5] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [6] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [7] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [8] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [9] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [10] | Li Hui, Chen Lianglong. Application and characteristics of bone graft materials in the treatment of spinal tuberculosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 626-630. |
| [11] | Gao Cangjian, Yang Zhen, Liu Shuyun, Li Hao, Fu Liwei, Zhao Tianyuan, Chen Wei, Liao Zhiyao, Li Pinxue, Sui Xiang, Guo Quanyi. Electrospinning for rotator cuff repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 637-642. |
| [12] | Guan Jian, Jia Yanfei, Zhang Baoxin , Zhao Guozhong. Application of 4D bioprinting in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 446-455. |
| [13] | Zeng Xinyu, Chen Xianghe, Liu Bo, Lu Pengcheng, Jin Shengjie, Li Wenxiu, Tian Zhikai, Sun Changliang. Mechanism of exercise improving bone metabolism in type 2 diabetics mellitus based on "Muscle-Bone" Crosstalk [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(2): 289-295. |
| [14] | Huang Bo, Chen Mingxue, Peng Liqing, Luo Xujiang, Li Huo, Wang Hao, Tian Qinyu, Lu Xiaobo, Liu Shuyun, Guo Quanyi . Fabrication and biocompatibility of injectable gelatin-methacryloyl/cartilage-derived matrix particles composite hydrogel scaffold [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2600-2606. |
| [15] | Liu Jiali, Suo Hairui, Yang Han, Wang Ling, Xu Mingen. Influence of lay-down angles on mechanical properties of three-dimensional printed polycaprolactone scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2612-2617. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||