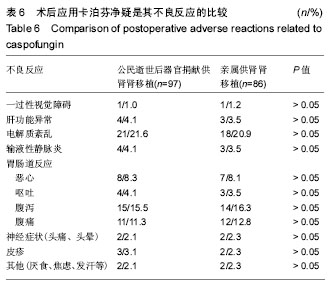
| [1] Veroux M, Corona D, Gagliano M,et al.Voriconazole in the treatment of invasive aspergillosis in kidney transplant recipients.Transplant Proc. 2007;39(6):1838-1840.[2] Pappas PG, Rex JH, Lee J,et al.A prospective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients.Clin Infect Dis. 2003;37(5): 634-643.[3] Pemán J, Cantón E, Gobernado M,et al.Epidemiology and antifungal susceptibility of Candida species isolated from blood: results of a 2-year multicentre study in Spain.Eur J Clin Microbiol Infect Dis. 2005;24(1):23-30.[4] Alexander J, Limaye AP, Ko CW,et al.Association of hepatic iron overload with invasive fungal infection in liver transplant recipients. Liver Transpl. 2006;12(12):1799-1804.[5] Kawagishi N, Satoh K, Enomoto Y,et al.Risk factors and impact of beta-D glucan on invasive fungal infection for the living donor liver transplant recipients.Tohoku J Exp Med. 2006;209(3):207-215.[6] Winkler M, Pratschke J, Schulz U,et al.Caspofungin for post solid organ transplant invasive fungal disease: results of a retrospective observational study.Transpl Infect Dis. 2010;12(3):230-237.[7] Koselj-Kajtna M, Kandus A, Rott T, et al.Aspergillus infection in immunocompromised patients.Transplant Proc. 2001;33(3): 2176-8217.[8] Shi SH, Lu AW, Shen Y,et al.Spectrum and risk factors for invasive candidiasis and non-Candida fungal infections after liver transplantation.Chin Med J (Engl). 2008;121(7):625-630.[9] Ju MK, Joo DJ, Kim SJ,et al.Invasive pulmonary aspergillosis after solid organ transplantation: diagnosis and treatment based on 28 years of transplantation experience.Transplant Proc. 2009;41(1): 375-378..[10] 中国心脏死亡器官捐献分类标准[OL].2017.http://www. moh.gov.cn/publicfiles///business/cmsresources/mohylfwjgs/cmsrsdocument/doc11852.doc[11] van Schaik RH, van der Heiden IP, van den Anker JN,et al.CYP3A5 variant allele frequencies in Dutch Caucasians.Clin Chem. 2002; 48(10):1668-1671.[12] De Pauw B, Walsh TJ, Donnelly JP,et al.Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group.Clin Infect Dis. 2008; 46(12):1813-1821.[13] Summers DM,Counter C,Johnson RJ,et al. Is the increase in DCD organ donors in the United Kingdom contributing to a decline in DBD donors? Transplantation. 2010; 90(12) :1506-1510.[14] Faraj W,Fakih H,Mukherji D,et al. Organ donation after cardiac death in the Middle East. Transplant Proc. 2010;42(3):713-715.[15] Harari S. Current strategies in the treatment of invasive Aspergillus infections in immunocompromised patients. Drugs. 1999;58(4): 621-631.[16] Theuretzbacher U. Pharmacokinetics/pharmacodynamics of echinocandins. Eur J Clin Microbiol Infect Dis. 2004;23(11):805-812.[17] Denning DW. Echinocandin antifungal drugs. Lancet. 2003;362(9390): 1142-1151.[18] Dupont BF,Lortholary O,Ostrosky-Zeichner L,et al. Treatment of candidemia and invasive candidiasis in the intensive care unit:post hoc analysis of a randomized,controlled trial comparing micafungin and liposomal amphotericin B.Critical Care.2009;13(5):R159.[19] Hashino S,Morita L,Takahata M,et al.Administration of micafungin as prophylactic antifungal therapy in patients undergoing allogeneic stem cell transplantation. Int J Hematol.2008;87(1):91-97.[20] Chen JS,Li LS. Cheng DR,et al. Effect of CYP3A5 genotype on renal allograft recipients treated with tacrolimus. Transplant Proc.2009;41(5): 1557-1561.[21] Kuehl P,Zhang J,Lin Y,et al.Sequence diversity in CYP3A promoters and characterization of thegenetic basis of polymorphic CYP3A5 expression.Nat Genet.2001;27(4):383-391. [22] Würthwein G, Cornely OA, Trame MN,et al.Population pharmacokinetics of escalating doses of caspofungin in a phase II study of patients with invasive aspergillosis.Antimicrob Agents Chemother. 2013;57(4):1664-1671. |