Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (29): 4748-4756.doi: 10.3969/j.issn.2095-4344.2017.29.026
A systematic review of stem cells in treatment of spinal cord injury and a network Meta-analysis of the therapeutic effects via different transplantation ways
Liu Ying, Liu Chu-fan, Zhang Hui-ting, Shi Ye, Lan Shan, Tang Ling-ling, Ai Jin-wei, Pei Bin
- Evidence-Based Medicine Center, Hubei University of Medicine, Xiangyang 442000, Hubei Province, China
-
Online:2017-10-18Published:2017-11-08 -
Contact:Pei Bin, Master’s supervisor, Chief physician, Professor, Evidence-Based Medicine Center, Hubei University of Medicine, Xiangyang 442000, Hubei Province, China -
About author:Liu Ying, Master, Associate chief nurse, Evidence-Based Medicine Center, Hubei University of Medicine, Xiangyang 442000, Hubei Province, China; Liu Chu-fan, Evidence-Based Medicine Center, Hubei University of Medicine, Xiangyang 442000, Hubei Province, China Liu Ying and Liu Chu-fan contributed equally to this work. -
Supported by:the General Program for Western Medicine in 2015-2016 Hubei Health and Family Planning, No. WJ2015MB187; the Teaching and Research Project of Hubei University of Medicine, No. 2015025; the Scientific Plan Project of Xiangyang City, No. 2010GG3A21
CLC Number:
Cite this article
Liu Ying, Liu Chu-fan, Zhang Hui-ting, Shi Ye, Lan Shan, Tang Ling-ling, Ai Jin-wei, Pei Bin. A systematic review of stem cells in treatment of spinal cord injury and a network Meta-analysis of the therapeutic effects via different transplantation ways[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(29): 4748-4756.
share this article
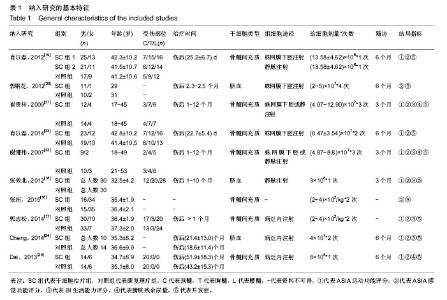
2.1 文献检索结果 从各数据库初检出文献7 465篇,Note Express3.1软件排除重复后4 346篇,经标题、摘要初筛获得可能纳入的文献53篇,再通过阅读全文复筛,最终纳入10个研究[16-25]。其中,中文文献8篇[16-23],英文文献2篇[24-25]。 2.2 纳入研究基本特征 纳入的10个临床随机对照试验涉及546例脊髓损伤患者,干细胞治疗组294例,综合康复理疗组252例。其中1个研究为3臂试验[19],因此本研究包含12个研究。干细胞治疗时间均在脊髓损伤后1个月以上,最长达10余年。3个研究运用脐血间充质干细胞[18,20,24],其余研究运用骨髓间充质干细胞。给细胞途径包括蛛网膜下腔注射、静脉注射、病灶内注射,2个研究描述为“经蛛网膜下腔或静脉注射”[21-22],1个研究未明确描述给细胞途径[16]。干细胞治疗次数界于1-4次,多数研究为2次。纳入研究的基本特征见表1。"


2.3 纳入研究方法质量学评价 纳入的10个随机临床对照试验质量评价见表2。按改良Jadad量表,3个研究得分3分[16,18,20],7个研究得分5分[17,19,21-25],10个研究平均分为4.40分,总体为高质量研究。 2.4 两种方法比较治疗脊髓损伤Meta分析结果 2.4.1 ASIA运动功能评分 9个研究报告了ASIA运动功能评分[17-19,21-25],合计样本量422例,干细胞治疗组232例,康复理疗组190例。5个研究报告了治疗1个月的ASIA运动功能评分[19,21-23],合并结果显示两组疗效差异无统计学意义[I2=0,MD=0.11,95%CI(-0.14,0.37)];7个研究报告了治疗3个月的ASIA运动功能评分[18-23],合并结果显示干细胞治疗组ASIA运动功能评分高于康复理疗组[I2=0,MD=0.21,95%CI(0.01,0.41)];7个研究报报告了治疗6个月的ASIA运动功能评分[16,19,20,23-25],合并结果显示干细胞治疗组ASIA运动功能评分高于康复理疗组[I2=0,MD=0.71,95%CI(0.50,0.92)],见图1。 2.4.2 ASIA感觉功能评分 11个研究报告了ASIA感觉功能评分[16-25],合计样本量546例,干细胞治疗组294例,康复理疗组252例。5个研究报告了治疗1个月的ASIA感觉功能评分[19,21-23],合并结果显示两组疗效差异无统计学意义[I2=0,MD=0.13,95%CI(-0.12,0.38)];7个研究报告了治疗3个月的ASIA感觉功能评分[17-19,21-23],合并结果显示干细胞治疗组ASIA感觉功能评分高于康复理疗组[I2=0.0%,MD=0.47,95%CI(0.27,0.68)];7个研究报告了治疗6个月的ASIA感觉功能评分[16,19-20,23-25],合并结果显示干细胞治疗组ASIA感觉功能评分高于康复理疗组[I2=29.7%,MD=0.66,95%CI(0.44,0.87)],见图2。 2.4.3 BI生活能力评分 7个研究报告BI生活能力评 分[16-18,20-22,24],合计样本量346例,干细胞治疗组169例,康复理疗组177例。2个研究报告了治疗1个月的BI生活能力评分[21-22],合并结果显示两组疗效差异无统计学意义[I2=0,MD=0.04,95%CI(-0.48,0.55)];4个研究报告了治疗3个月的BI生活能力评分[17-18,21-22],合并结果显示干细胞治疗组BI生活能力评分高于康复理疗组[I2=4.2%,MD=0.57,95%CI(0.28,0.85)];3个研究报告了治疗6个月的BI生活能力评分[16,20,24],合并结果显示干细胞治疗组BI生活能力评分高于康复理疗组[I2=0,MD=0.57,95%CI(0.24,0.90)],见图3。"


2.4.4 膀胱残余尿量 3个研究报告了膀胱残余尿量[21-22,25],合计样本量98例,干细胞治疗组47例,康复理疗组51例。2个研究报告了治疗1个月的膀胱残余尿量[21-22],合并结果显示两组疗效差异无统计学意义[I2=0,MD=-0.40,95%CI(-0.92,0.12)];2个研究报告了治疗3个月的膀胱残余尿量[21- 22],合并结果显示干细胞治疗组膀胱残余尿量少于康复理疗组[I2=41%,MD=-0.58,95%CI(-1.11,-0.05)];1个研究报告了治疗6个月的膀胱残余尿量[25],合并结果显示干细胞治疗组膀胱残余尿量少于康复理疗组[MD=-1.00,95%CI(-1.66,-0.34)] ,见图4。 2.4.5 并发症发生率 8个研究报告了治疗过程中并发症率[17-19,21-25],合计样本量386例,干细胞治疗组79例,康复理疗组81例。并发症主要包括低热、腹胀、头痛、下肢麻木、脑膜刺激征等。Meta分析结果显示,干细胞治疗过程中发热(低热)发生率为14%[95%CI(5%,22%)],腹胀发生率为7% [95%CI(0,17%)],头痛腹胀发生率为7%[95%CI (1%,12%)],下肢麻木(蛛网膜下腔注射)发生率为8% [95%CI (1%,15%)],脑膜刺激征(蛛网膜下腔注射)发生率为7% [95%CI(3%,17%)]。 2.5 不同移植途径疗效比较Meta分析结果 2个研究注射方法描述为“蛛网膜下腔注射或静脉注射”[21-22],1个研究未描述具体细胞移植途径[16],予以排除。1个研究为3臂试验[19],故含9个两两比较。以ASIA运动功能评分、ASIA感觉功能评分、BI生活能力评分和并发症发生率为结局指标,以综合康复理疗为共同对照进行网状Meta分析。干预措施间比较证据网络图以ASIA运动功能评分为例见图5。因纳入比较数量较少,未行小样本效应检测。各结局指标证据网络图均形成1个“康复理疗-蛛网膜下腔注射-静脉注射”三角形闭环,IF下限均为0,说明闭环一致性好。MCMC拟合一致模型模型结果显示:3种干细胞移植途径间比较,在ASIA运动功能评分、ASIA感觉功能评分、BI生活能力评分和并发症率方面差异均无统计学意义,见表3,4;与康复理疗比较,仅干细胞蛛网膜下腔注射在改善ASIA运动功能评分[MD=9.77,95%CI(0.26,21.46)优于综合康复理疗。运用MCMC拟合不一致模型进行敏感性分析,各治疗措施间比较结果未发生明显变化,说明本研究结果是稳定的。"

| [1] Hagen EM.Acute complications of spinal cord injuries.World J Orthop.2015;6(1):17-23.[2] Merghati Khoi E,Latifi S,Rahdari F,et al.The effect of injury-related characteristics on changes in marital status after spinal cord injury.Iran J Public Health.2015;44(10): 1395-1402.[3] Dedeepiya VD,William JB,Parthiban JK,et al.The known-unknowns in spinal cord injury, with emphasis on cell-based therapies - a review with suggestive arenas for research.Expert Opin Biol Ther.2014;14(5):617-634.[4] Dolbow DR,Gorgey AS,Recio AC,et al.Activity-based restorative therapies after spinal cord injury: inter-institutional conceptions and perceptions.Aging Dis.2015;6(4):254-261.[5] Hagen EM,Rekand T.Management of neuropathic pain associated with spinal cord injury. Pain Ther.2015;4(1):51-65.[6] Y?lmaz T,Kaptano?lu E.Current and future medical therapeutic strategies for the functional repair of spinal cord injury.World J Orthop.2015;6(1):42-55.[7] Sezer N,Akku? S,U?urlu FG.Chronic complications of spinal cord injury.World J Orthop.2015; 6(1):24-33.[8] Wang J,Pearse DD.Therapeutic hypothermia in spinal cord injury: the status of its use and open questions.Int J Mol Sci.2015;16(8):16848-16879.[9] Schroeder GD,Kepler CK,Vaccaro AR.The Use of Cell Transplantation in Spinal Cord Injuries. J Am Acad Orthop Surg.2016;24(4):266-275.[10] Goel A.Stem cell therapy in spinal cord injury: Hollow promise or promising science? J Craniovertebr Junction Spine. 2016; 7(2):121-126.[11] Marichal N,Fabbiani G,Trujillo-Cenoz O,et al.Purinergic signalling in a latent stem cell niche of the rat spinal cord. Purinergic Signal.2016;12(2):331-341.[12] Guo L,Rolfe AJ,Wang X,et al.Rescuing macrophage normal function in spinal cord injury with embryonic stem cell conditioned media.Mol Brain.2016;9(1):48.[13] Neirinckx V,Agirman G,Coste C,et al.Adult bone marrow mesenchymal and neural crest stem cells are chemoattractive and accelerate motor recovery in a mouse model of spinal cord injury. Stem Cell Res Ther.2015;6:211.[14] Li J,Lepski G.Cell transplantation for spinal cord injury: a systematic review.Biomed Res Int.2013;2013:786475.[15] Ades AE,Caldwell DM,Reken S,et al.Evidence synthesis for decision making 7: a reviewer's checklist.Med Decis Making. 2013;33(5):679-691.[16] 张拓,佟旭,刘晨.骨髓间充质干细胞对脉冲低剂量效率放射性脊髓损伤细胞修复作用的研究[J].世界最新医学信息文摘,2015, 15(A0):64-65.[17] 郭志松,秦秉玉,代荣钦,等.骨髓问充质干细胞治疗脊髓损伤的研究[J].中华实验外科杂志,2014, 31(11):2605-2607.[18] 张效北,李江涛,赵和泰,等.间质干细胞治疗脊髓损伤的临床分析[J].亚太传统医药,2012,8(3):116-117.[19] 肖以磊,朱建新,李忠民,等.两种不同途径移植自体骨髓间充质干细胞治疗早期脊髓损伤疗效观察[J].中国医师进修杂志,2012, 35(14):24-28.[20] 郭钢花,申利坊,李哲.脐血间充质干细胞治疗脊髓损伤临床研究[J].中国实用医刊,2012, 39(10):58-60.[21] 崔贵祥,宋成忠,李义召,等.自体骨髓单个核细胞治疗脊髓损伤的临床研究[J].中国康复医学杂志, 2009,24(4):304-312.[22] 谢遵伟,崔贵祥,李义召,等.自体骨髓间充质干细胞治疗脊髓损伤疗效观察[J].中国组织工程研究与临床康复,2007,11(7):1277-1279.[23] 肖以磊,李忠民,朱建新,等.自体骨髓间充质干细胞治疗早期脊髓损伤疗效观察[J].中华生物医学工程杂志,2014,20(1):7-11.[24] Cheng H,Liu X,Hua R,et al.Clinical observation of umbilical cord mesenchymal stem cell transplantation in treatment for sequelae of thoracolumbar spinal cord injury.J Transl Med. 2014; 12:254.[25] Dai G,Liu X,Zhang Z,et al.Transplantation of autologous bone marrow mesenchymal stem cells in the treatment of complete and chronic cervical spinal cord injury. Brain Res.2013; 1533: 73-79.[26] 赵琳,杨彦玲,韩继明.细胞移植及细胞联合移植修复脊髓损伤的研究进展[J].神经解剖学杂志,2014,30(5):605-608.[27] 彭岱,王汉东.干细胞在脊髓损伤治疗现状与进展[J].医学研究生学报,2013,26(11):1226-1229.[28] Alkabie S,Boileau AJ.The role of therapeutic hypothermia after traumatic spinal cord injury-A systematic review.World Neurosurg.2016;86:432-449.[29] Gomez-Villafuertes R.Contribution of purinergic receptors to spinal cord injury repair: stem cell-based neuroregeneration. Neural Regen Res.2016;11(3):418-419.[30] Pati S,Pilia M,Grimsley JM,et al.Cellular therapies in trauma and critical care medicine: forging new frontiers. Shock. 2015;44(6):505-523.[31] Batista CE,Mariano ED,Marie SK,et al. Stem cells in neurology - current perspectives.Arq Neuropsiquiatr. 2014;72(6):457-465.[32] Lin XY,Lai BQ,Chen MT,et al.Cell transplantation and neuroengineering approach for spinal cord injury treatment: a summary of current laboratory findings and review of literature. Cell Transplant.2016;25(8):1425-1438.[33] Myers SA,Bankston AN,Burke DA,et al.Does the preclinical evidence for functional remyelination following myelinating cell engraftment into the injured spinal cord support progression to clinical trials? Exp Neurol.2016;283(Pt B): 560-572.[34] Mendonca MV,Larocca TF,de Freitas SB,et al.Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury.Stem Cell Res Ther.2014;5(6):126.[35] Ra JC,Shin IS,Kim SH,et al.Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans.Stem Cells Dev.2011;20(8):1297-1308.[36] Lima C,Pratas-Vital J,Escada P,et al.Olfactory mucosa autografts in human spinal cord injury: a pilot clinical study.J Spinal Cord Med.2006;29(3):191-203.[37] Shroff G.Human embryonic stem cell therapy in chronic spinal cord injury: A retrospective study. Clin Transl Sci.2016;9(3): 168-175.[38] Cheng I,Githens M,Smith RL,et al.Local versus distal transplantation of human neural stem cells following chronic spinal cord injury.Spine J.2016;16(6):764-769.[39] Oh SK,Choi KH,Yoo JY,et al.A Phase III Clinical Trial Showing Limited Efficacy of Autologous Mesenchymal Stem Cell Therapy for Spinal Cord Injury. Neurosurgery. 2016;78(3): 436-447.[40] Oraee-Yazdani S,Hafizi M,Atashi A,et al.Co-transplantation of autologous bone marrow mesenchymal stem cells and Schwann cells through cerebral spinal fluid for the treatment of patients with chronic spinal cord injury: safety and possible outcome.Spinal Cord.2016; 54(2):102-109.[41] Lin XY,Lai BQ,Zeng X,et al.Cell transplantation and neuroengineering approach for spinal cord injury treatment: A summary of current laboratory findings and review of literature.Cell Transplant.2016;25(8):1425-1438.[42] Aras Y,Sabanci PA,Kabatas S,et al.The effects of adipose tissue-derived mesenchymal stem cell transplantation during the acute and subacute phases following spinal cord injury. Turk Neurosurg.2016;26(1):127-139. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | Jing Jinpeng, Zhang Yue, Liu Xiaomin, Liu Yi. Traditional Chinese medicine injection for promoting blood circulation in prevention of deep vein thrombosis after orthopedic surgery: network meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1467-1476. |
| [3] | Xiang Xinjian, Liu Fang, Wu Liangliang, Jia Daping, Tao Yue, Zhao Zhengnan, Zhao Yu. High-dose vitamin C promotes the survival of autologous fat transplantation in rats [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1242-1246. |
| [4] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [5] | Xiao Hao, Liu Jing, Zhou Jun. Research progress of pulsed electromagnetic field in the treatment of postmenopausal osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1266-1271. |
| [6] | Liu Gang, Ma Chao, Wang Le, Zeng Jie, Jiao Yong, Zhao Yi, Ren Jingpei, Hu Chuanyu, Xu Lin, Mu Xiaohong. Ankle-foot orthoses improve motor function of children with cerebral palsy: a Meta-analysis based on 12 randomized controlled trials [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1299-1304. |
| [7] | Wu Min, Zhang Yeting, Wang Lu, Wang Junwei, Jin Yu, Shan Jixin, Bai Bingyi, Yuan Qiongjia. Effect of concurrent training sequences on body composition and hormone response: a Meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1305-1312. |
| [8] | Fang Xiaolei, Leng Jun, Zhang Chen, Liu Huimin, Guo Wen. Systematic evaluation of different therapeutic effects of mesenchymal stem cell transplantation in the treatment of ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1085-1092. |
| [9] | Guo Jia, Ding Qionghua, Liu Ze, Lü Siyi, Zhou Quancheng, Gao Yuhua, Bai Chunyu. Biological characteristics and immunoregulation of exosomes derived from mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1093-1101. |
| [10] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [11] | Huang Chenwei, Fei Yankang, Zhu Mengmei, Li Penghao, Yu Bing. Important role of glutathione in stemness and regulation of stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1119-1124. |
| [12] | Hou Jingying, Guo Tianzhu, Yu Menglei, Long Huibao, Wu Hao. Hypoxia preconditioning targets and downregulates miR-195 and promotes bone marrow mesenchymal stem cell survival and pro-angiogenic potential by activating MALAT1 [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1005-1011. |
| [13] | Zhou Ying, Zhang Huan, Liao Song, Hu Fanqi, Yi Jing, Liu Yubin, Jin Jide. Immunomodulatory effects of deferoxamine and interferon gamma on human dental pulp stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1012-1019. |
| [14] | Liang Xuezhen, Yang Xi, Li Jiacheng, Luo Di, Xu Bo, Li Gang. Bushen Huoxue capsule regulates osteogenic and adipogenic differentiation of rat bone marrow mesenchymal stem cells via Hedgehog signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1020-1026. |
| [15] | Wang Jifang, Bao Zhen, Qiao Yahong. miR-206 regulates EVI1 gene expression and cell biological behavior in stem cells of small cell lung cancer [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1027-1031. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||