Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (27): 4397-4402.doi: 10.3969/j.issn.2095-4344.2017.27.023
Previous Articles Next Articles
The latest research on the pathogenesis of osteonecrosis of the femoral head
Ma Jian-xiong, He Wei-wei, Zhao Jie, Ma Xin-long
- Orthopedic Institute of Tianjin Hospital, Tianjin 300211, China
-
Online:2017-09-28Published:2017-10-24 -
Contact:Ma Xin-long, Master, Professor, Orthopedic Institute of Tianjin Hospital, Tianjin 300211, China -
About author:Ma Jian-xiong, M.D., Associate researcher, Orthopedic Institute of Tianjin Hospital, Tianjin 300211, China -
Supported by:the State Key Research Development Program, No. 2016YFC0105805; the National Natural Science Foundation of China, No. 81673994 and 81571723; the Key Technologies Research and Development Program, No. 2014KY31
CLC Number:
Cite this article
Ma Jian-xiong, He Wei-wei, Zhao Jie, Ma Xin-long . The latest research on the pathogenesis of osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2017, 21(27): 4397-4402.
share this article
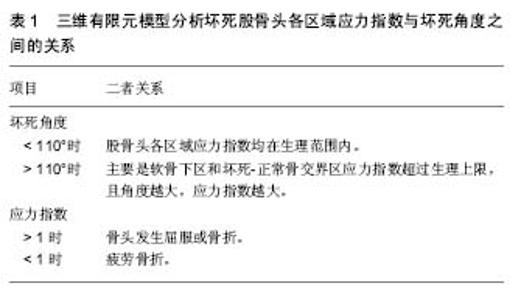
2.1 血运障碍 关于股骨头坏死发病机制的假说很多,但很多学者普遍认为股骨头坏死的最终共同通路是股骨头的血运损害,Chandle认为股骨头的解剖和心脏十分相似,将股骨头坏死称作“髋关节的冠心病”,很多研究中甚至将股骨头坏死称为股骨头缺血性坏死(avascular necrosis of the femoral head,ANFH)[14-16]。Liu等[16]通过数字减影血管造影观察股骨头坏死患者股骨头血供情况,结果显示:57(48.31%)髋有上支持带动脉损伤,21(17.80%)髋有下支持带动脉损伤,40 (33.90%)髋上、下支持动脉均有损伤;相比于下支持带动脉损伤,上支持带动脉损伤的股骨头更容易且更快进展至股骨头塌陷。认为股骨头塌陷主要是上支持带动脉损伤所导致。有学者分析股骨颈骨折患者股骨头血供变化,也有类似发现[17]。 创伤性髋关节脱位可损伤支持带血管和圆韧带血管,使血供突然中断,导致相应区域的股骨头失去营养支持。不少学者进一步深入研究导致股骨头缺血的具体机制。Feng等[18]发现非创伤性股骨头坏死患者外周血中内皮祖细胞的数量及功能均下降,而内皮祖细胞与人体的血管修复及新生血管形成密切相关。体内促凝与抗凝活动的平衡是血流学稳定的基础,凝血障碍被认为与股骨头坏死密切相关[9-10]。 有研究报道非创伤性股骨头坏死患者的血管假性血友病因子(vWF)及纤溶酶原激活物抑制因子(PAI-1)水平显著高于正常人[19]。Glueck等[20]研究股骨头坏死患者血栓形成情况和纤溶状况,发现特发性股骨头坏死患者的凝血因子Ⅷ和低纤溶性脂蛋白a水平比健康人群高,而继发性股骨头坏死患者中,凝血因子Ⅴ、Ⅷ和抗活化蛋白C水平更高。对此,加拿大学者Séguin等[21]研究发现了相悖结果:与普通人群相比,非创伤性股骨头坏死患者并未发现任何一种特定的血栓形成因子(抗凝血酶Ⅲ、蛋白C、蛋白S等)异常。但有学者发现股骨头血流分布与坏死区域并不相符[22],对血供障碍导致股骨头坏死提出了质疑。Nakamura等[23]研究用糖皮质激素治疗的系统性红斑狼疮患者股骨头血供情况,研究显示患者股骨头血供并未减少,尤其是15岁以下的小儿患者股骨头血供仍然十分丰富;而最终却是股骨头血供十分丰富的小儿患者出现了股骨头坏死。这与传统的血运障碍学说相悖,且人体具有很强的修复能力,短期缺血能够得以重建或形成侧支循环。Kim等[24]对特发性股骨头坏死患者进行随访并分析患者灌注MRI资料,与初次诊断时相比,之后从股骨头骨骺的内侧、外侧及后侧向中央区域逐渐发生血管重建,血流灌注显著增加。临床上,股骨颈骨折患者往往是在负重行走后1-3年内出现股骨头坏死,如果单纯是缺血导致股骨头坏死,解释不了其出现症状的时间。 2.2 骨细胞凋亡 人的软骨细胞、骨细胞、成骨细胞和破骨细胞上均存在糖皮质激素受体,糖皮质激素与受体结合,引起构象改变,进而导致相应转录因子转位或阻抑。Rojas等[25]对肾移植患者移植前后分别进行骨活检,发现移植前无成骨细胞凋亡,移植之后有大量成骨细胞凋亡,且大部分未凋亡的成骨细胞由活性状态转变为无活性状态,而这些现象与患者糖皮质激素积累量呈显著相关。有研究分析对比股骨头坏死患者及患其他髋部疾病患者关节置换术后股骨头标本[26],原位末端标记(TUNEL)结果显示,坏死股骨头内骨细胞及成骨细胞凋亡率显著高于非坏死股骨头。 越来越多学者认识到股骨头坏死中骨细胞凋亡的存在,骨细胞凋亡过程中具体调控机制也被大家所重视。Wnt信号通路在胚胎发育及细胞凋亡调控方面发挥着重要作用。Zhang等[27]通过动物实验发现:糖皮质激素通过减少Wnt通路下游基因活性β-catenin及c-Myc表达,导致骨细胞及成骨细胞的凋亡,进而引起早期激素性股骨头坏死。DKK1是一种Wnt通路抑制剂,能调控骨组织的发育,抑制DKK1的表达能减轻糖皮质激素引起的骨量丢失和成骨细胞凋亡[28]。 Ko等[29]对比分析股骨头坏死患者和股骨颈骨折患者股骨头中DKK1、Bad、Bcl2、TNFα、Wnt3a、LRP5以及Axin1表达情况。结果显示激素性和酒精性股骨头坏死患者股骨头内:成骨细胞和纤维母细胞大量凋亡,且这两种细胞中DKK1呈高表达;DKK1和Bad(一种促凋亡调控子)的mRNA表达量显著增加;血清DKK1水平升高,且越晚期患者血清DKK1水平越高。对DKK1进行RNA干扰之后,DKK1、Bad的mRNA表达量和凋亡细胞量显著减少,而人重组DKK1蛋白使Bad的mRNA表达量和凋亡细胞量均增加。激素服用及酗酒促进了DKK1的表达,进而引起大量骨细胞凋亡,股骨头坏死进展。 近年来,小分子核糖核酸(microRNA)越来越被学者所重视[30-32]。MicroRNA参与转录后基因表达调控,调节着近三分之一的人类基因[33],对人体骨的调节也发挥着重要作用[34]。通过动物实验,Liu等[35]发现Cx43/microRNA- 206 对DKK1和Wnt信号通路表达和骨细胞凋亡的调控可能与早期股骨头坏死发生密切相关。 除了经典的Wnt信号通路,其他信号通路也被发现与股骨头坏死骨细胞凋亡密切相关。Xu等[36]研究不同阶段激素性股骨头坏死患者骨细胞凋亡情况:相比于早期患者,晚期股骨头坏死患者骨细胞凋亡现象更严重,且STAT1及caspase 3表达量随之增加,STAT1-caspase 3信号通路在激素性股骨头坏死发生发展中发挥着重要作用。 Wnt信号通路和STAT1-caspase 3信号通路对骨细胞凋亡的调控,影响着股骨头坏死的发生和发展。 2.3 基因多态性 1986年,Hall[37]研究发现Legg-Calvé- Perthes(LCP)病先证者的一级亲属中,该病的发病率为2.5%,是普通人群的35倍,意味着存在影响LCP病易感性的基因。Miyamoto等[38]对日本一个存在多名家庭成员患有LCP病的家族研究,发现其Ⅱ型胶原纤维基因COL2A1存在错义突变,该突变(p.G1170S)使编码的氨基酸发生改变,扰乱了Ⅱ型胶原纤维的基本结构。Miyamoto认为COL2A1基因突变在家族性LCP病的家庭成员中较为常见。 Chen等[39]研究两个LCP病家系遗传信息,发现家族性LCP病为常染色体显性遗传,并将候选基因定位在染色体12q13上D12S1663及D12S1632之间的15-cM区域内。为进一步找出相关基因,Chen等[40]对比分析3个LCP病家系中32名患LCP病成员和65名散发型股骨头坏死患者的染色体12q13上相应区域的遗传信息,发现3个LCP病家系中两个家系中的患LCP病成员的COL2A1基因的外显子50出现G→A突变,另一个家系中的患LCP病成员的COL2A1基因的外显子33出现G→A突变;而65例散发型股骨头坏死患者的COL2A1基因未发现突变。COL2A1基因突变可能为遗传性股骨头坏死的根本原因。Kannu等[41]在2例LCP患儿身上也检测到COL2A1基因的突变。COL2A1基因突变与LCP疾病密切相关。 韩国学者Hwang等[42]对特发性股骨头坏死及酒精性股骨头坏死患者血清,利用比较基因组杂交技术及实时聚合酶链反应技术(RT-PCR)分析其遗传信息,虽未能明确导致股骨头坏死的确切基因,但发现 FLJ40296、CYP27C1及CTDP1这3个基因出现频率最高(均> 50%)。Wang等[43]对比分析209例酒精性股骨头坏死患者与300名正常人的ApoA1与ApoB基因的单核苷酸多态性(SNP),结果显示ApoB基因中 ,rs1042034、rs676210 及rs673548这3种SNP能降低酒精性股骨头坏死的风险,而ApoA1基因中rs632153能增加酒精性股骨头坏死的风险。ApoA1及ApoB基因多态性与酒精性股骨头坏死相关。Balla等[44]利用基于TaqMan探针的RT-PCR技术分析股骨头坏死患者与无股骨头坏死的健康人骨组织中基因表达的差别,通过多变量数据分析发现9个基因(SP7,COL1A2,COL5A2,COL10A1,ITGA2,MMP10,TNC,KL,VEGF)的表达存在显著差异。 特发性股骨头坏死存在家族聚集和遗传现象,而非特发性股骨头坏死也存在基因异常。明确股骨头坏死的遗传原因,有助于疾病的早期预测、预防和诊治。 2.4 生物力学因素 根据wolff定律,骨骼能承受骨组织的机械应变,并具有适应这些功能需要的能力。强调了结构和功能的统一,骨结构决定其功能,而一定的功能又需要相应的骨结构去实现。髋关节作为全身主要承重关节,日常活动中股骨头承受着较大应力。对此,有些学者认为股骨头坏死并不是缺血所导致的,而是骨结构和功能不统一,生物力学因素所导致的。 Ueo等[45]发现股骨头坏死区域与其血管分布情况并不一致,大部分患者坏死区域呈球面凸状,认为这意味着生物力学因素在股骨头坏死发展过程中起重要作用。通过有限元分析,Ueo提出了自己的观点:最开始由于股骨头内血管堵塞导致一小部分区域骨坏死,坏死骨和正常松质骨是两种不同材质,在这两者交界处产生应力集中,超过其生理范围,引起机械破坏,如此恶性循环,导致更大范围的骨坏死。 韩国学者Yang等[46]利用三维有限元模型分析坏死股骨头各区域应力指数S(有效应力/屈服强度)和坏死角度的关系(表1),有限元分析显示无论坏死角多大,股骨头各区域应力指数均小于1。因此,可认为股骨头坏死塌陷是疲劳骨折所致。对28例股骨头坏死标本观察发现:19个(68%)标本中骨折位于坏死区深部紧邻坏死-正常交界区,7个(25%)标本中骨折位于软骨下区,2个(7%)标本中上述两个区域均有骨折,与有限元分析结果一致。股骨头坏死发展过程中,其内部骨小梁的生物力学特性逐渐发生改变。"
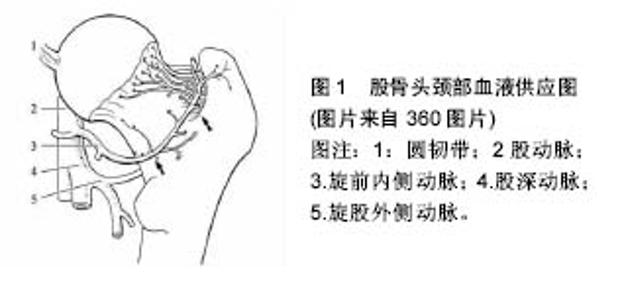
Kim等[47]分别测试塌陷前期、塌陷期、塌陷晚期患者股骨头的无血管区及其底下区域力学性能,结果显示:塌陷前期患者股骨头两区域的弹性模量、屈服强度和极限强度均无差异;塌陷期患者股骨头无血管区上述指标均小于其底下区域;塌陷晚期患者股骨头无血管区极限强度高于其底下区域;塌陷期和塌陷晚期患者软骨下区及深部无血管与反应交界区可见到大裂隙。有学者分析股骨头坏死患者坏死区域分布规律,发现股骨头坏死主要分布在股骨头应力集中区域,而极少分布在血供集中区域。如果是缺血导致股骨头坏死,股骨头坏死的分布应与股骨头血液供应分布相符(股骨头血液供应分别见图1)。 Daniel等[48]通过建立数学模型观察到:当坏死区承重能力下降、坏死区变大或者坏死区越靠外侧,髋关节接触应力会显著增加;且若坏死区位于负重较少的区域,髋关节接触应力几乎不受影响。 作者认为髋关节应力增加是股骨头坏死进展为骨性关节炎的重要原因。各种危险因素作用下,股骨头承受应力能力下降,而外界机械应力不断刺激累积,内部骨小梁发生疲劳微骨折,产生应力集中,内部结构更易受损,如此恶性循环,机体在此过程中不断修复,当修复无法抵挡外界应力刺激时,股骨头发生塌陷。 硬化带是股骨头修复的一个产物,Yu等[49]通过有限元分析得出:硬化带能为股骨头提供有效的力学支持,为坏死组织提供力学保护,减少股骨头变形,延缓或防止塌陷。石少辉[50]研究发现:有硬化带形成患者出现髋关节疼痛到关节置换时间为(49±11)个月,显著长于无硬化带形成患者(15±2)个月。股骨头坏死的临床治疗中,骨移植治疗能给病变股骨头提供支撑,恢复应力传导,避免应力集中,提供了一个稳定的力学环境,能有效防止股骨头塌陷。"
| [1] Moya-Angeler J, Gianakos AL, Villa JC, et al. Current concepts on osteonecrosis of the femoral head. World J Orthop.2015; 6(8): 590-601.[2] Koch PP,Tannast M,Fujita H,et al.Minimum ten year results of total hip arthroplasty with the acetabular reinforcement ring in avascular osteonecrosis. Int Orthop.2008; 32(2): 173-179.[3] Zhao DW, Yu M, Hu K, et al.Prevalence of Nontraumatic Osteonecrosis of the Femoral Head and its Associated Risk Factors in the Chinese Population: Results from a Nationally Representative Survey. Chin Med J (Engl).2015;128(21): 2843-2850.[4] Amanatullah DF, Strauss EJ, Di Cesare PE.Current management options for osteonecrosis of the femoral head: part 1, diagnosis and nonoperative management. Am J Orthop (Belle Mead NJ).2011;40(9): E186-192.[5] Mont M, Cherian J, Sierra R, et al. Nontraumatic Osteonecrosis of the Femoral Head: Where Do We Stand Today? A Ten-Year Update. J Bone Joint Surg Am.2015; 97(19): 1604-1627.[6] Guerado E, Caso E. The physiopathology of avascular necrosis of the femoral head: an update. Injury.2016;47 Suppl 6: S16-S26.[7] Kumar MN, Belehalli P, Ramachandra P. PET/CT study of temporal variations in blood flow to the femoral head following low-energy fracture of the femoral neck. Orthopedics.2014; 37(6): e563-570.[8] Wang G, Zhang CQ, Sun Y, et al. Changes in femoral head blood supply and vascular endothelial growth factor in rabbits with steroid-induced osteonecrosis. J Int Med Res. 2010; 38(3):1060-1069.[9] Zeng X, Zhan K, Zhang L, et al. The impact of high total cholesterol and high low-density lipoprotein on avascular necrosis of the femoral head in low-energy femoral neck fractures. J Orthop Surg Res.2017;12(1): 30.[10] Mukisi M, Bashoun K, Burny F. Sickle-cell hip necrosis and intraosseous pressure. Orthop Traumatol Surg Res.2009; 95(2):134-138.[11] Jilka RL, Noble B, Weinstein RS. Osteocyte apoptosis. Bone. 2013; 54(2): 264-271.[12] Zheng L, Wang W, Ni J, et al. The association of eNOS gene polymorphism with avascular necrosis of femoral head. PloS one.2014;9(2): e87583.[13] Tian L, Wen Q, Dang X, et al. Immune response associated with Toll-like receptor 4 signaling pathway leads to steroid-induced femoral head osteonecrosis. BMC Musculoskeletal Disorders.2014;15(1): 18.[14] Lau RL, Perruccio AV, Evans HM, et al.Stem cell therapy for the treatment of early stage avascular necrosis of the femoral head: a systematic review. BMC Musculoskelet Disord. 2014; 15:156.[15] Liu BY, Zhao DW, Yu XB, et al. Effect of superior retinacular artery damage on osteonecrosis of the femoral head. Chin Med J (Engl).2013;126(20): 3845-3850.[16] Rupp RE, Rupp SN. Femoral Head Avascular Necrosis Is Not Caused by Arthroscopic Posterolateral Femoroplasty. Orthopedics.2016;39(3): 177-180.[17] 申锋,阎作勤,郭常安,等. SPECT-CT评价股骨颈骨折后股骨头血供的变化[J]. 中华关节外科杂志:电子版, 2012, 6(2): 6-8.[18] Feng Y, Yang SH, Xiao BJ, et al.Decreased in the number and function of circulation endothelial progenitor cells in patients with avascular necrosis of the femoral head. Bone.2010;46(1): 32-40.[19] Li C, Shen L,Yang Y, et al.Plasma ghrelin and von Willebrand Factor levels in patients with non-traumatic osteonecrosis of the femoral head. Hip Int.2015;25(1): 76-81.[20] Glueck CJ, Freiberg RA, Wang P. Heritable thrombophilia- hypofibrinolysis and osteonecrosis of the femoral head. Clin Orthop Relat Res.2008;466(5): 1034-1040.[21] Seguin C,Kassis J,Busque L,et al.Non-traumatic necrosis of bone (osteonecrosis) is associated with endothelial cell activation but not thrombophilia. Rheumatology (Oxford). 2008;47(8): 1151-1155.[22] 廖速成. 成人股骨头坏死的应力机制研究[D].广州中医药大学, 2015.[23] Nakamura J, Ohtori S, Watanabe A, et al. Recovery of the blood flow around the femoral head during early corticosteroid therapy: dynamic magnetic resonance imaging in systemic lupus erythematosus patients. Lupus.2012;21(3): 264-270.[24] Kim HK,Burgess J,Thoveson A,et al.Assessment of Femoral Head Revascularization in Legg-Calve-Perthes Disease Using Serial Perfusion MRI.J Bone Joint Surg Am.2016; 98(22):1897-1904.[25] Rojas E, Carlini RG, Clesca P, et al. The pathogenesis of osteodystrophy after renal transplantation as detected by early alterations in bone remodeling. Kidney Int.2003;63(5): 1915-1923.[26] Mutijima E, De Maertelaer V, Deprez M, et al. The apoptosis of osteoblasts and osteocytes in femoral head osteonecrosis: its specificity and its distribution. Clin Rheumatol.2014;33(12): 1791-1795.[27] Zhang C, Zou Y L, Ma J, et al. Apoptosis associated with Wnt/beta-catenin pathway leads to steroid-induced avascular necrosis of femoral head. BMC Musculoskelet Disord. 2015; 16:132.[28] Wang FS,Ko JY,Yeh DW,et al.Modulation of Dickkopf-1 attenuates glucocorticoid induction of osteoblast apoptosis, adipocytic differentiation, and bone mass loss. Endocrinology. 2008;149(4): 1793-1801.[29] Ko JY, Wang FS, Wang CJ, et al. Increased Dickkopf-1 expression accelerates bone cell apoptosis in femoral head osteonecrosis. Bone.2010;46(3): 584-591.[30] Hansen T, Jensen T, Clausen B, et al. Natural RNA circles function as efficient microRNA sponges. Nature.2013; 495(7441): 384-388.[31] Ha M, Kim V. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol.2014;15(8): 509-524.[32] Lewis B, Burge C, Bartel D. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1): 15-20.[33] Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov.2017;16(3): 203-222.[34] Moore BT, Xiao P. MiRNAs in bone diseases. MicroRNA. 2013;2(1): 20-31.[35] Liu G, Luo G, Bo Z, et al. Impaired osteogenic differentiation associated with connexin43/microRNA-206 in steroid-induced avascular necrosis of the femoral head. Exp Mol Pathol. 2016; 101(1): 89-99.[36] Xu X, Wen H, Hu Y, et al. STAT1-caspase 3 pathway in the apoptotic process associated with steroid-induced necrosis of the femoral head. J Mol Histol.2014;45(4):473-485.[37] Hall DJ. Genetic aspects of Perthes' disease. A critical review. Clin Orthop Relat Res.1986;(209):100-114.[38] Miyamoto Y, Matsuda T, Kitoh H, et al.A recurrent mutation in type II collagen gene causes Legg-Calve-Perthes disease in a Japanese family. Hum Genet. 2007;121(5): 625-629.[39] Chen WM, Liu YF, Lin MW, et al.Autosomal dominant avascular necrosis of femoral head in two Taiwanese pedigrees and linkage to chromosome 12q13. Am J Hum Genet.2004; 75(2): 310-317.[40] Liu YF, Chen W M, Lin Y F, et al. Type II collagen gene variants and inherited osteonecrosis of the femoral head. N Engl J Med.2005;352(22): 2294-2301.[41] Kannu P, Irving M, Aftimos S, et al. Two novel COL2A1 mutations associated with a Legg-Calve-Perthes disease-like presentation. Clin Orthop Relat Res.2011; 469(6): 1785-1890.[42] Hwang J T, Baik SH, Choi JS, et al. Genetic traits of avascular necrosis of the femoral head analyzed by array comparative genomic hybridization and real-time polymerase chain reaction. Orthopedics.2011;34(1): 14.[43] Wang Y, Cao Y, Li Y, et al. Genetic association of the ApoB and ApoA1 gene polymorphisms with the risk for alcohol-induced osteonecrosis of femoral head. Int J Clin Exp Pathol.2015; 8(9): 11332-11339.[44] Balla B, Pinter C, Kosa J P, et al. Gene expression changes in femoral head necrosis of human bone tissue. Dis Markers. 2011;31(1): 25-32.[45] Ueo T,Tsutsumi S,Yamamuro T,et al. Biomechanical aspects of the development of aseptic necrosis of the femoral head.Arch Orthop Trauma Surg.1985;104(3): 145-149.[46] Yang JW, Koo KH, Lee MC, et al.Mechanics of femoral head osteonecrosis using three-dimensional finite element method . Arch Orthop Trauma Surg.2002;122(2): 88-92.[47] Kim YM, Lee SH, Lee FY,et al.Morphologic and biomechanical study of avascular necrosis of the femoral head. Orthopedics.1991;14(10): 1111-1116.[48] Daniel M,Herman S,Dolinar D,et al.Contact stress in hips with osteonecrosis of the femoral head. Clin Orthop Relat Res. 2006;447:92-99.[49] Yu T, Xie L, Chu F. A sclerotic rim provides mechanical support for the femoral head in osteonecrosis. Orthopedics. 2015;38(5): e374-9.[50] 石少辉,李子荣,王佰亮,等. 激素性股骨头坏死硬化带与骨形态蛋白关系的研究[J]. 中华外科杂志, 2010, 48(17): 1305-1308.[51] Gao YS, Guo SC, Ding H, et al. Caspase-3 may be employed as an early predictor for fractureinduced osteonecrosis of the femoral head in a canine model. Mol Med Rep.2012;6(3): 611-614.[52] Jia YB, Jiang DM, Ren YZ, et al. Inhibitory effects of vitamin E on osteocyte apoptosis and DNA oxidative damage in bone marrow hemopoietic cells at early stage of steroid-induced femoral head necrosis. Mol Med Rep.2017;15(4): 1585-1592.[53] Wang J, Kalhor A, Lu S, et al. iNOS expression and osteocyte apoptosis in idiopathic, non-traumatic osteonecrosis. Acta Orthop.2015;86(1): 134-141.[54] Bai R,Feng W, Liu W L, et al. Roles of osteocyte apoptosis in steroid-induced avascular necrosis of the femoral head. Genet Mol Res.2016;15(1). [55] Weinstein R S. Glucocorticoid-induced osteonecrosis. Endocrine.2012;41(2): 183-190. |
| [1] | Wang Jianping, Zhang Xiaohui, Yu Jinwei, Wei Shaoliang, Zhang Xinmin, Xu Xingxin, Qu Haijun. Application of knee joint motion analysis in machanism based on three-dimensional image registration and coordinate transformation [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(在线): 1-5. |
| [2] | Zhang Yufang, Lü Meng, Mei Zhao. Construction and verification of a full spine biomechanical model of adolescent scoliosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1351-1356. |
| [3] | Bai Zixing, Cao Xuhan, Sun Chengyi, Yang Yanjun, Chen Si, Wen Jianmin, Lin Xinxiao, Sun Weidong. Construction and biomechanical analysis of ankle joint finite element model in gait cycle [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1362-1366. |
| [4] | Liu Feng, Feng Yi. Finite element analysis of different Kirschner wire tension bands on transverse patella fractures during gait cycle [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1367-1371. |
| [5] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [6] | Jin Tao, Liu Lin, Zhu Xiaoyan, Shi Yucong, Niu Jianxiong, Zhang Tongtong, Wu Shujin, Yang Qingshan. Osteoarthritis and mitochondrial abnormalities [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1452-1458. |
| [7] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [8] | Luo Xiaoling, Zhang Li, Yang Maohua, Xu Jie, Xu Xiaomei. Effect of naringenin on osteogenic differentiation of human periodontal ligament stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1051-1056. |
| [9] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [10] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [11] | Hou Jingying, Guo Tianzhu, Yu Menglei, Long Huibao, Wu Hao. Hypoxia preconditioning targets and downregulates miR-195 and promotes bone marrow mesenchymal stem cell survival and pro-angiogenic potential by activating MALAT1 [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1005-1011. |
| [12] | Wen Mingtao, Liang Xuezhen, Li Jiacheng, Xu Bo, Li Gang. Mechanical stability of Sanders II type calcaneal fractures fixed by two internal fixation methods [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(6): 838-842. |
| [13] | Huang Hao, Hong Song, Wa Qingde. Finite element analysis of the effect of femoral component rotation on patellofemoral joint contact pressure in total knee arthroplasty [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(6): 848-852. |
| [14] | Zheng Yongze, Zheng Liqin, He Xingpeng, Chen Xinmin, Li Musheng, Li Pengfei, Lin Ziling. Extended finite element modeling analysis of femoral neck fracture based on ABAQUS software [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(6): 853-857. |
| [15] | Liu Yuhang, Zhou Jianqiang, Xu Xuebin, Qu Xingyue, Li Ziyu, Li Kun, Wang Xing, Li Zhijun, Li Xiaohe, Zhang Shaojie. Establishment and validation of finite element model of lower cervical spine in 6-year-old children [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(6): 870-874. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||