Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (26): 4185-4191.doi: 10.3969/j.issn.2095-4344.2017.26.015
Previous Articles Next Articles
Preparation, characterization and performance of gamma-polyglutamic acid/carboxymethyl chitosan-calcium phosphate cement
- Guangdong Institute of Microbiology, Guangzhou 510070, Guangdong Province, China
-
Received:2017-04-15Online:2017-09-18Published:2017-09-28 -
Contact:Shi Qing-shan, Master’s supervisor, Guangdong Institute of Microbiology, Guangzhou 510070, Guangdong Province, China -
About author:Shu Xiu-lin, Master, Associate investigator, Guangdong Institute of Microbiology, Guangzhou 510070, Guangdong Province, China -
Supported by:the Science and Technology Planning Project of Guangdong Province, No. 2013B010102016, and the Natural Science Foundation of Guangdong Province, No. 2014A030313665
CLC Number:
Cite this article
Shu Xiu-lin, Shi Qing-shan, Chen Ming-jie, Feng Jin.
share this article
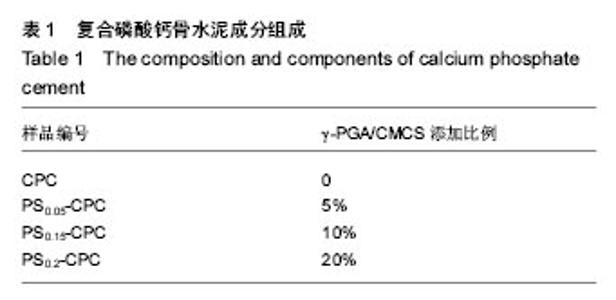
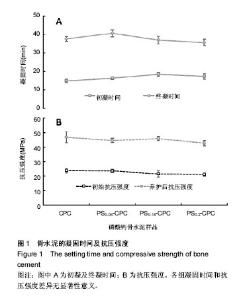
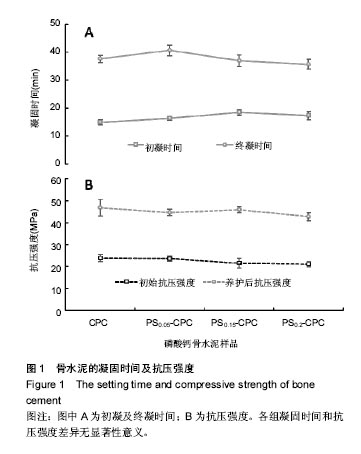
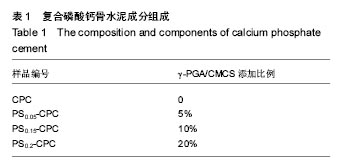
2.1 γ-PGA/CMCS复合材料对凝固时间的影响 各组分CPC的添加成分比例见表1,添加γ-PGA/CMCS颗粒对CPC凝结时间的影响见图1。从表1和图1A中可见,随着 γ-PGA/CMCS颗粒的增加,CPC的凝结时间逐渐延长,对照CPC初凝和终凝时间分别为(15.0±0.9) min和(37.6±1.3) min,γ-PGA/CMCS的加入延长了CPC初凝时间,但是终凝时间反而有所缩短,γ-PGA/CMCS颗粒添加量越多,终凝时间越短,如当γ-PGA/CMCS添加量为20%时,其初凝和终凝时间分别为(17.4±1.4) min和(35.7±1.7) min。根据CPC凝结过程的物理本质,其凝结时间取决于使固体颗粒间距缩短至产生化学键力连接时所需水化产物的总量与产物成核生长速率的相对比值。由于γ- PGA/CMCS 颗粒为吸水性凝胶体,在CPC基体中贮存吸收部分固化液体,使实际液固比下降,导致各颗粒间距离缩短,因而减少终凝时间。"

凝固时间是可注射骨水泥临床应用的重要指标之一,初凝时间过长,在与生理体液接触时,骨再生水泥浆体容易被体液或者血液冲散,从而形成血栓,给患者带来风险;初凝时间太短,则没有充足的手术时间[7-8]。因此选择适合的凝固时间对骨水泥的实际应用非常重要。 γ-PGA/CMCS 含量对养护前后CPC抗压强度的影响见图1B。对照CPC初始抗压强度为(23.97±1.59) MPa,养护3 d后对照CPC抗压强度为(46.76±3.82) MPa。随着 γ-PGA/CMCS颗粒的加入,骨水泥的初始抗压强度和最终抗压强度均有所降低,γ-PGA/CMCS添加量越多,其初始抗压强度越小,与对照CPC相比均未达到显著差异水平。但是经过3 d养护后,γ-PGA/CMCS的添加量为15%试件的抗压强度反而最大,为(45.81±1.38) MPa,最小抗压强度为(42.731±1.82) MPa(γ-PGA/CMCS比例为20%)。这可能是由于养护后γ-PGA/CMCS在CPC基体中吸水力强,干燥后形成较多空隙,当外加压力时,界面出现应力集中现象,达到极限值,结构便迅速遭受破坏,显示较大的脆性。 综上,由于PS0.15-CPC具有稍长的初凝时间和较快的终凝时间,而且经过养护后其抗压强度与对照CPC相接近,所以选定PS0.15-CPC作为试验样品进行下一步研究。 2.2 复合CPC微观形态 复合CPC养护72 h后的微观结构见图2。图2A为未养护对照CPC,图2B为养护后的对照CPC;图2C和2D为γ-PGA/CMCS复合的骨水泥样品(PS-CPC),其中图2C为未养护的PS-CPC,图2D为养护后的PS-CPC。从图2中可以看出,相比无规则晶体形态的对照CPC,未养护的PS0.15-CPC中CPC基体呈现规则片状晶体,而且片状晶体之间清晰可见γ-PGA/CMCS颗粒的存在,并且γ-PGA/CMCS颗粒较均匀的分布在CPC片状晶体中间(图2C)。经过养护后,相比对照CPC,PS0.15-CPC中没有明显的γ-PGA/CMCS颗粒单独存在,2种试件表面上有大量的片状结晶或是被片状结晶包裹住。结果可见,复合CPC在γ-PGA/CMCS颗粒与骨水泥基体结合的界面处发生了局部的水化反应,与CPC无明显分界线,使界面结合更加牢固。"

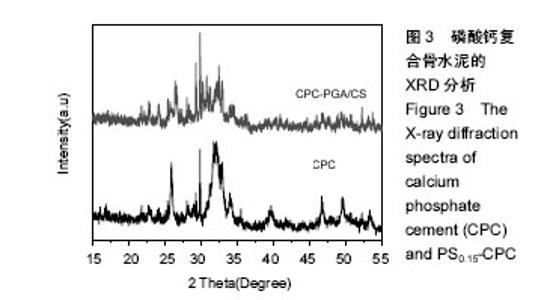
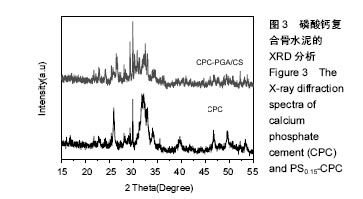
2.3 复合磷酸钙骨水泥的物相分析,X衍射光分析 图3 显示了CPC和PS0.15-CPC支架的XRD特征。2种支架含有羟基磷灰石的特定峰值(25.4°,25.8°,28.2°,29.3°,29.9°,32.5°,33°,46.7°,49.5°)。除此之外,与纯CPC相比,PS-CPC特定高峰还集中在26.5°,31.1°,35.4°,45.9°,52.5°。这些结果表明,γ-PGA/CMCS的合并没有参与CPC对羟基磷灰石的变换,改性后CPC主要晶型不变,但是结晶度有所降低。据研究,低结晶度磷酸钙具有较快的降解速度、良好的生物活性及生物相容性,可以较好地黏附细胞并促进其增殖,引导成骨细胞生长,从而具有更加广泛的应用范围。"
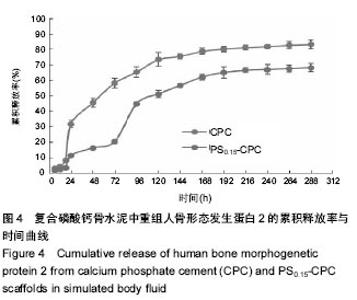
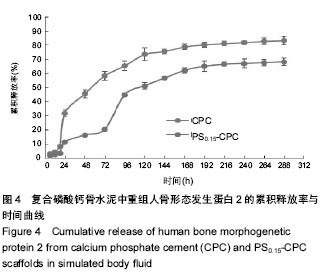
2.4 复合磷酸钙骨水泥载体药物体外模拟释放 图4是CPC直接载入重组人骨形态发生蛋白2的F-CPC试件的药物释放图。从图4可见,无论在对照F-CPC还是复合F-PS-CPC载体中,药物释放存在前期突释和缓慢释放2个阶段。在对照F-CPC中,重组人骨形态发生蛋白2的快速突释发生在24 h,突释量都在32%左右;之后药物释放较为缓慢并趋于稳定,12 d释放量达83%。由于重组人骨形态发生蛋白2通过与CPC固相粉末共混载入F-CPC,因此均匀分布在CPC中,重组人骨形态发生蛋白2与CPC分子间没有强的化学键形成。大量的研究显示F-CPC是通过扩散控制药物释放的,随着载药量的增加,药物在基质表面及内部的分布增多,导致了前期快速释放量的增多以及基质孔隙率的增大,最终导致了药物释放量和速率的提高。 对于γ-PGA/CMCS复合F-PS-CPC载体而言,其药物释放前期存在2个突释期,前期突释时间大概为24 h,但是释放量仅为16.5%;此后一直是缓慢释放,但是到了96 h,又发生了第二次突释放,释放量达到50%,这是因为随着药物的释放和γ-PGA/CMCS凝胶体的降解,基质孔隙率随着载入量的增加而增大,导致了药物释放量和速率的提高。相比同期对照CPC的重组人骨形态发生蛋白2的释放量,复合CPC的释放相对较低;之后一段时间药物释放接近于线性释放趋势,8 d后释放变缓慢,12 d后释放率达68%。γ-PGA/CMCS装载重组人骨形态发生蛋白2的药物释放相对于直接共混载入CPC而言起到了明显的缓释效果。"
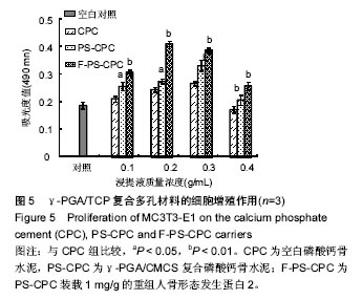
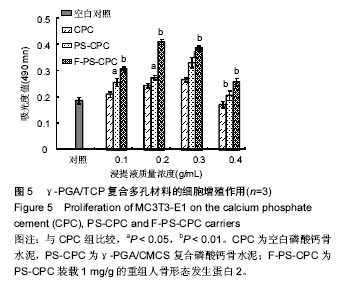
2.5 细胞相容性实验 成骨细胞(MC3T3-E1)在3种试件材料浸提液中均生长良好,细胞折光性较好,贴壁紧密,未见细胞碎裂现象。从图5可以看出,MTS实验表明CPC各组材料浸提液培养MC3T3-E1 2 d后,各组试件的吸光度值均高于空白对照组,说明各试件对MC3T3-E1均无体外细胞毒性。在不同的浸提液浓度下,与对照CPC相比,PS-CPC复合材料对细胞增殖有显著促进作用(P < 0.05),这可能是由于其粗糙的表面以及规则的拓扑结构有利于细胞的增殖(图2);但是当浸提液质量浓度达到0.4 g/mL时对细胞无促进作用;当PS-CPC负载生长因子(F-PS-CPC)后,其对细胞均有促增殖作用(P < 0.01),甚至当浸提液质量浓度0.4 g/mL时依然对细胞有一定促增殖作用,且与对照CPC相比差异有显著性意义(P < 0.05),但是与空白对照相比,质量浓度为0.4 g/mL的浸提液样品对细胞增殖无明显促进作用,可见控制载体材料的降解速率对细胞增殖有重要意义。MTS结果表示,PS-CPC复合材料能促进成骨细胞的生长和增殖,表现出良好的细胞相容性。"
| [1]Reyes R, De la Riva B, Delgado A, et al. Effect of triple growth factor controlled delivery by a brushite-PLGA system on a bone defect. Injury. 2012;43(3):334-342.[2]Makhdom AM, Hamdy RC. The role of growth factors on acceleration of bone regeneration during distraction osteogenesis. Tissue Eng Part B Rev. 2013;19(5): 442-453.[3]Vo TN, Kasper FK, Mikos AG. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv Drug Deliv Rev. 2012;64(12):1292-1309.[4]Khojasteh A, Behnia H, Naghdi N, et al. Effects of different growth factors and carriers on bone regeneration: a systematic review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(6):e405-423.[5]Zhang X, Cresswell M. Calcium Phosphate Materials for Controlled Release Systems. Inorganic Controlled Release Technology. 2016:167-187.[6]Ginebra MP, Traykova T, Planell JA. Calcium phosphate cements as bone drug delivery systems: a review. J Control Release. 2006;113(2):102-110.[7]李理,李百川,王仁崇,等.新型氯化锂复合磷酸钙骨水泥的理化性能及成骨特性[J].中国组织工程研究, 2016,20(3):307-313.[8]Schummer W, Schlonski O, Breuer M. Bone cement embolism attached to central venous catheter. Br J Anaesth. 2014;112(4):672-674.[9]Zhang J, Liu W, Schnitzler V, et al. Calcium phosphate cements for bone substitution: chemistry, handling and mechanical properties. Acta Biomater. 2014;10(3): 1035-1049.[10]Shu X, Shi Q, Feng J, et al. Design and in vitro evaluation of novel γ-PGA/hydroxyapatite nanocomposites for bone tissue engineering. J Mater Sci. 2014; 49(22):7742-7749.[11]Shu XL, Shi QS, Feng J, et al. Poly (γ-glutamic acid)/beta-TCP nanocomposites via in situ copolymerization: Preparation and characterization. J Biomater Appl. 2016; 31(1):102-111.[12]Ginebra MP, Canal C, Espanol M, et al. Calcium phosphate cements as drug delivery materials. Adv Drug Deliv Rev. 2012; 64(12):1090-1110.[13]潘朝晖.壳聚糖纤维及明胶增强的磷酸钙骨水泥治疗骨缺损的实验研究[D].西安:第四军医大学, 2006.[14]Takagi S, Chow LC, Hirayama S, et al. Properties of elastomeric calcium phosphate cement-chitosan composites. Dent Mater. 2003;19(8):797-804.[15]Aryaei A, Liu J, Jayatissa AH, et al. Cross-linked chitosan improves the mechanical properties of calcium phosphate-chitosan cement. Mater Sci Eng C Mater Biol Appl. 2015;54:14-19.[16]Meng D, Dong L, Wen Y, et al. Effects of adding resorbable chitosan microspheres to calcium phosphate cements for bone regeneration. Mater Sci Eng C Mater Biol Appl. 2015; 47:266-272.[17]Park KH, Kim SJ, Lee WY, et al. Hydrothermal fabrication and characterization of calcium phosphate anhydrous/chitosan composites. Ceramics International. 2017; 43(2): 2786-2790.[18]Rochet N, Balaguer T, Boukhechba F, et al. Differentiation and activity of human preosteoclasts on chitosan enriched calcium phosphate cement. Biomaterials. 2009;30(26):4260-4267.[19]Takechi M, Miyamoto Y, Momota Y, et al. The in vitro antibiotic release from anti-washout apatite cement using chitosan. J Mater Sci Mater Med. 2002;13(10):973-978.[20]疏秀林,施庆珊,林小平,等. γ-聚谷氨酸/壳聚糖多孔复合支架材料的制备、表征及性能的研究[J].天然产物研究与开发, 2013, 25(4): 514-518.[21]疏秀林,施庆珊,冯静,等. γ-聚谷氨酸发酵培养基的Plackett- Burman 法优化[J].生物技术通报,2007(4): 173-177.[22]疏秀林,施庆珊,冯静,等. 一株非谷氨酸依赖型聚 γ-谷氨酸高产菌株的鉴定与诱变育种[J].微生物学通报, 2009, 36(5): 705-710.[23]Sun Y, Liu Y, Liu W, et al. Chitosan microparticles ionically cross-linked with poly(γ-glutamic acid) as antimicrobial peptides and nitric oxide delivery systems. Biochem Eng J. 2015; 95:78-85.[24]Teixeira GQ, Leite Pereira C, Castro F, et al. Anti-inflammatory Chitosan/Poly-γ-glutamic acid nanoparticles control inflammation while remodeling extracellular matrix in degenerated intervertebral disc. Acta Biomater. 2016;42: 168-179.[25]Keresztessy Z, Bodnár M, Ber E, et al. Self-assembling chitosan/poly-γ-glutamic acid nanoparticles for targeted drug delivery. Colloid & Polymer Science.2009;287(7):759-765.[26]Liao ZX, Peng SF, Chiu YL, et al. Enhancement of efficiency of chitosan-based complexes for gene transfection with poly(γ-glutamic acid) by augmenting their cellular uptake and intracellular unpackage. J Control Release. 2014;193: 304-315.[27]Chung RJ, Ou KL, Tseng WK, et al. Controlled release of BMP-2 by chitosan/γ-PGA polyelectrolyte multilayers coating on titanium alloy promotes osteogenic differentiation in rat bone-marrow mesenchymal stem cells. Surface & Coatings Technology. 2016; 303:283-288.[28]Charles LF, Woodman JL, Ueno D, et al. Effects of low dose FGF-2 and BMP-2 on healing of calvarial defects in old mice. Exp Gerontol. 2015;64:62-69.[29]Driessens FM, Boltong MG, Bermudez O, et al. Formulation and setting times of some calcium orthophosphate cements: a pilot study. Journal of Materials Science: Materials in Medicine. 1993; 4(5): 503-508.[30]Kim YH, Tabata Y. Dual-controlled release system of drugs for bone regeneration. Advanced Drug Delivery Reviews. 2015; 94:28-40. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [3] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [4] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [5] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [6] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [7] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| [8] | Li Hui, Chen Lianglong. Application and characteristics of bone graft materials in the treatment of spinal tuberculosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 626-630. |
| [9] | Gao Cangjian, Yang Zhen, Liu Shuyun, Li Hao, Fu Liwei, Zhao Tianyuan, Chen Wei, Liao Zhiyao, Li Pinxue, Sui Xiang, Guo Quanyi. Electrospinning for rotator cuff repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 637-642. |
| [10] | Le Guoping, Zhang Ming, Xi Licheng, Luo Hanwen. Preparation and in vitro evaluation of vancomycin hydrochloride@polylactic acid-glycolic acid copolymer-chitosan-hyaluronic acid composite sustained-release microspheres [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 528-534. |
| [11] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| [12] | He Guanyu, Xu Baoshan, Du Lilong, Zhang Tongxing, Huo Zhenxin, Shen Li. Biomimetic orientated microchannel annulus fibrosus scaffold constructed by silk fibroin [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 560-566. |
| [13] | Guan Jian, Jia Yanfei, Zhang Baoxin , Zhao Guozhong. Application of 4D bioprinting in tissue engineering [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(3): 446-455. |
| [14] | Liu Jiali, Suo Hairui, Yang Han, Wang Ling, Xu Mingen. Influence of lay-down angles on mechanical properties of three-dimensional printed polycaprolactone scaffolds [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2612-2617. |
| [15] | Huang Bo, Chen Mingxue, Peng Liqing, Luo Xujiang, Li Huo, Wang Hao, Tian Qinyu, Lu Xiaobo, Liu Shuyun, Guo Quanyi . Fabrication and biocompatibility of injectable gelatin-methacryloyl/cartilage-derived matrix particles composite hydrogel scaffold [J]. Chinese Journal of Tissue Engineering Research, 2022, 10(16): 2600-2606. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||