Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (24): 3924-3930.doi: 10.3969/j.issn.2095-4344.2017.24.026
Previous Articles Next Articles
Bone-cartilage crosstalk: a conversation for understanding the pathogenesis and new treatment strategy of osteoarthritis
Li Guang-guang1, Guo Yang1, Dai Guo-da2, Ge Wen-jie2 , Ma Yong1, Yuan Han1, Dong Wu-xun1, Cai Jian-ping2
- 1Institute of Traumatology, Nanjing University of Chinese Medicine, Nanjing 210023, Jiangsu Province, China; 2Department of Traumatic Orthopedics, Wuxi Hospital of Traditional Chinese Medicine, Wuxi 214071, Jiangsu Province, China
-
Revised:2017-05-20Online:2017-08-28Published:2017-08-30 -
Contact:Cai Jian-ping, M.D., Chief physician, Department of Traumatic Orthopedics, Wuxi Hospital of Traditional Chinese Medicine, Wuxi 214071, Jiangsu Province, China -
About author:Li Guang-guang, Studying for master’s degree, Institute of Traumatology, Nanjing University of Chinese Medicine, Nanjing 210023, Jiangsu Province, China -
Supported by:the Science and Technology Project of Jiangsu Province, No. BK20151007; the Research Project of the Management Center of Wuxi Hospital, No. YGZXM14046; the Research Project of the Health and Family Planning Commission of Wuxi, No. Q201503; the Postgraduate Research Innovation Program of Universities in Jiangsu Province, No. KYZZ16_0409
CLC Number:
Cite this article
Li Guang-guang, Guo Yang, Dai Guo-da, Ge Wen-jie, Ma Yong, Yuan Han, Dong Wu-xun, Cai Jian-ping. Bone-cartilage crosstalk: a conversation for understanding the pathogenesis and new treatment strategy of osteoarthritis[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(24): 3924-3930.
share this article
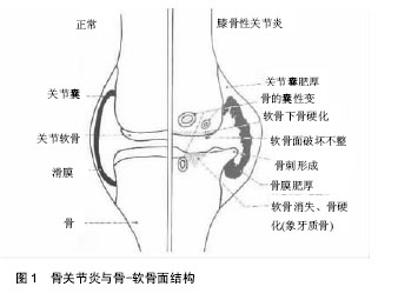
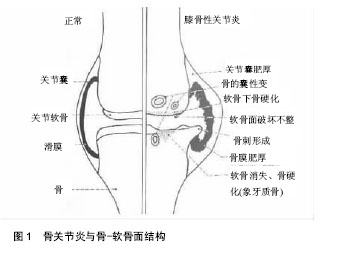
2.1 骨关节炎与骨-软骨面交互作用 在骨关节炎的进程中,骨软骨的微观结构发生了许多改变,包括软骨的丢失,软骨下骨的增厚,软骨下骨小梁数量的减少,关节边缘骨质增生,骨囊肿,骨髓病损和骨赘的形成[3](图1),以及潮线模糊,钙化软骨层增厚,软骨与软骨下骨间缝隙的扩大,软骨细胞增殖异常,血管微结构的改变等[6]。在骨关节炎疾病的发生发展阶段,关节软骨结构完整性的缺失以及生理功能的衰退与基质的分子组成改变有关。软骨细胞促进分子基质合成的同时,也促进了如白细胞介素1等促炎因子以及基质金属蛋白酶、聚蛋白多糖等组织破坏酶的产生[7]。血管内皮生长因子、人类转录因子以及基质金属蛋白酶13等因子可以导致软骨细胞肥大及细胞终末分化,这些因子的表达增加将引起细胞广泛变化[8]。细胞的异化活动将导致细胞基质重建,软骨构成物的丢失,软骨的钙化、龟裂,并引起软骨细胞的增殖、群集,降解特定基质蛋白以及细胞外基质的蛋白酶产物增加。最终,软骨面变得粗糙,并伴随软骨厚度变薄和纤维化[5, 9]。"

成骨细胞和破骨细胞分别引起骨基质形成和退化,在非正常机械负荷下,两者之间的平衡被破坏,引起了软骨下骨的重塑[10]。由此可见,骨代谢失衡可能是骨关节炎形成的重要发病机制。在病变早期,破骨细胞数量增多,骨质大量丢失,骨小梁厚度降低,骨重建发生速率增快,软骨下骨板缓慢增厚,最终导致软骨完全骨化[11]。软骨下骨板孔率的增高和厚度的变薄也与软骨损伤密切相关。在骨关节炎病变后期,成骨细胞活跃,促进骨的形成,增加了骨的密度和体积,导致软骨下骨明显变硬[12]。而在病变末期,软骨下骨厚度增加,硬度降低,钙化作用降低[12-13]。 软骨下骨病损是骨关节炎发生发展的风向标,也被认为是引起软骨结构恶化的重要风险因素[14]。骨重建激活局部骨组织自我修复功能,以增加相应的机械负荷,这与病变的发展是一致的。骨体积分数的增加、骨密度的降低以及骨小梁厚度的增加,可代偿关节区域机械活动,但也更容易增加该区域的摩擦[13]。骨髓病损的发生与局部软骨大量丢失有关,骨小梁微观结构的变化可以引起骨髓局部纤维化。利用MRI检测骨髓水肿和软骨下骨囊肿是评估骨关节炎病变的有效依据[15]。 破骨细胞过度活跃会在软骨下骨中扩展出一条通道,并最终通过骨垢线到达软骨下骨。血管再生和感觉神经的生长是紧密相连的整体过程,在骨关节炎的病变过程中,它与分子间增加的交互作用存在潜在联系。血管侵入的区域所形成的通道是由该区域软骨下骨的骨髓被血管内皮生长因子表达的维管组织取代,同时炎性细胞渗透至骨髓中所致[16]。此外,伴随着血管数量的增加,交感神经以及感觉神经从软骨下骨中延伸到骨-软骨通道中,并入侵正常软骨以提高血管通道内神经生长因子的表达[4]。动物模型实验表明,在骨关节炎病变早期,软骨下骨中血管生成活动更加频繁,并伴有血管侵入骨软骨连接处的现象[17]。 同时,运输通道(血管通道或是骨髓腔)的增加破坏了骨软骨面,提高了分子克服细胞外基质形成的屏障在关节间穿透的能力。有研究运用光漂白方法,发现在骨关节炎鼠模型中荧光素钠从钙化的软骨扩散到骨软骨面的速率比正常的关节更快,软骨损伤及血管侵入都能够增加扩散的渠道,提高小分子的交互作用[18]。在骨关节炎的病变过程中,骨转换也促进了软骨和软骨下骨之间的交互作用。骨关节炎鼠模型研究的结果显示,在病变早期,随着软骨下骨小梁、骨髓细胞以及关节软骨的生物化学和生物力学的交互作用,软骨下骨板孔隙逐渐增多。研究表明,骨重建发生速率的增快及受其刺激产生的血管因子是骨关节炎发生发展不可或缺的因素[11, 19]。 2.2 骨-软骨交互的细胞相互作用 见表1。大量的体外研究表明,软骨细胞、成骨细胞、破骨细胞及骨细胞之间存在紧密的分子交互用[20]。实验结果显示了在人工环境中这些细胞相互作用的潜在机制,并为后续的体内模型试验提供基础。Gabay等[21]发现来自骨关节炎患者软骨下骨的软骨细胞,其合成代谢的分子产物明显增加且表型发生改变。而且,Leyh等认为骨关节炎患者的软骨细胞分泌的多种细胞因子和生长因子促进软骨蛋白多糖的丢失[22]。"

| [1] Taylor AM, Hsueh MF, Ranganath LR, et al. Cartilage biomarkers in the osteoarthropathy of alkaptonuria reveal low turnover and accelerated ageing. Rheumatology (Oxford). 2017. DoI: 10.1093/rheumatology/kew355.[2] Goldring SR. Alterations in periarticular bone and cross talk between subchondral bone and articular cartilage in osteoarthritis. Ther Adv Musculoskelet Dis. 2012;4(4):249-258.[3] Barreto G, Soininen A, Ylinen P, et al. Soluble biglycan: a potential mediator of cartilage degradation in osteoarthritis. Arthritis Res Ther. 2015;17:379.[4] Cornejo MC, Cho SK, Giannarelli C, et al. Soluble factors from the notochordal-rich intervertebral disc inhibit endothelial cell invasion and vessel formation in the presence and absence of pro-inflammatory cytokines. Osteoarthritis Cartilage. 2015;23(3):487-496.[5] Ramme AJ, Lendhey M, Raya JG, et al. A novel rat model for subchondral microdamage in acute knee injury: a potential mechanism in post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2016;24(10):1776-1785.[6] Henrotin Y, Pesesse L, Sanchez C. Subchondral bone and osteoarthritis: biological and cellular aspects. Osteoporos Int. 2012;23 Suppl 8:S847-S851.[7] Novak T, Fites GK, Xu X, et al. In Vivo Cellular Infiltration and Remodeling in a Decellularized Ovine Osteochondral Allograft. Tissue Eng Part A. 2016;22(21-22):1274-1285.[8] Filip A, Pinzano A, Bianchi A, et al. Expression of the semicarbazide-sensitive amine oxidase in articular cartilage: its role in terminal differentiation of chondrocytes in rat and human. Osteoarthritis Cartilage. 2016;24(7):1223-1234.[9] Dore DA, Winzenberg TM, Ding C, et al. The association between objectively measured physical activity and knee structural change using MRI. Ann Rheum Dis. 2013;72(7): 1170-1175.[10] Madden RM, Han SK, Herzog W. The effect of compressive loading magnitude on in situ chondrocyte calcium signaling. Biomech Model Mechanobiol. 2015;14(1):135-142.[11] Pritchard JM, Papaioannou A, Tomowich C, et al. Bone mineralization is elevated and less heterogeneous in adults with type 2 diabetes and osteoarthritis compared to controls with osteoarthritis alone. Bone. 2013;54(1):76-82.[12] Chen Y, Wang T, Guan M, et al. Bone turnover and articular cartilage differences localized to subchondral cysts in knees with advanced osteoarthritis. Osteoarthritis Cartilage. 2015; 23(12):2174-2183.[13] Steinbeck MJ, Eisenhauer PT, Maltenfort MG, et al. Identifying Patient-Specific Pathology in Osteoarthritis Development Based on MicroCT Analysis of Subchondral Trabecular Bone. J Arthroplasty. 2016;31(1):269-277.[14] Sergijenko A, Roelofs AJ, Riemen AH, et al. Bone marrow contribution to synovial hyperplasia following joint surface injury. Arthritis Res Ther. 2016;18:166.[15] Muratovic D, Cicuttini F, Wluka A, et al. Bone marrow lesions detected by specific combination of MRI sequences are associated with severity of osteochondral degeneration. Arthritis Res Ther. 2016;18:54.[16] Moon HJ, Yurube T, Lozito TP, et al. Effects of secreted factors in culture medium of annulus fibrosus cells on microvascular endothelial cells: elucidating the possible pathomechanisms of matrix degradation and nerve in-growth in disc degeneration. Osteoarthritis Cartilage. 2014;22(2):344-354.[17] Saito M, Sasho T, Yamaguchi S, et al. Angiogenic activity of subchondral bone during the progression of osteoarthritis in a rabbit anterior cruciate ligament transection model. Osteoarthritis Cartilage. 2012;20(12):1574-1582.[18] Wang B, Zhou X, Price C, et al. Quantifying load-induced solute transport and solute-matrix interaction within the osteocyte lacunar-canalicular system. J Bone Miner Res. 2013;28(5): 1075-1086.[19] Shabestari M, Vik J, Reseland JE, et al. Bone marrow lesions in hip osteoarthritis are characterized by increased bone turnover and enhanced angiogenesis. Osteoarthritis Cartilage. 2016; 24(10):1745-1752.[20] Tanaka Y. Human mesenchymal stem cells as a tool for joint repair in rheumatoid arthritis. Clin Exp Rheumatol. 2015;33 (4 Suppl 92):S58-S62.[21] Gabay O, Zaal K J, Sanchez C, et al. Sirt1-deficient mice exhibit an altered cartilage phenotype. Joint Bone Spine. 2013; 80(6):613-620.[22] Leyh M, Seitz A, Durselen L, et al. Subchondral bone influences chondrogenic differentiation and collagen production of human bone marrow-derived mesenchymal stem cells and articular chondrocytes. Arthritis Res Ther. 2014;16(5):453.[23] Das RH, Jahr H, Verhaar JA, et al. In vitro expansion affects the response of chondrocytes to mechanical stimulation. Osteoarthritis Cartilage. 2008;16(3):385-391.[24] Khattab HM, Aoyama E, Kubota S, et al. Physical interaction of CCN2 with diverse growth factors involved in chondrocyte differentiation during endochondral ossification. J Cell Commun Signal. 2015;9(3):247-254.[25] Hayashi J, Kihara M, Kato H, et al. A proteomic profile of synoviocyte lesions microdissected from formalin-fixed paraffin-embedded synovial tissues of rheumatoid arthritis. Clin Proteomics. 2015;12(1):20.[26] Prasadam I, Farnaghi S, Feng J Q, et al. Impact of extracellular matrix derived from osteoarthritis subchondral bone osteoblasts on osteocytes: role of integrinbeta1 and focal adhesion kinase signaling cues. Arthritis Res Ther. 2013;15(5):R150.[27] Sharma AR, Jagga S, Lee SS, et al. Interplay between cartilage and subchondral bone contributing to pathogenesis of osteoarthritis. Int J Mol Sci. 2013;14(10):19805-19830.[28] Bolon B, Grisanti M, Villasenor K, et al. Generalized Degenerative Joint Disease in Osteoprotegerin (Opg) Null Mutant Mice. Vet Pathol. 2015;52(5):873-882.[29] Chen S, Huang Y, Chen W, et al. Protective effects of the Tougu Xiaotong capsule on morphology and osteoprotegerin/nuclear factor-kappaB ligand expression in rabbits with knee osteoarthritis. Mol Med Rep. 2016;13(1): 419-425.[30] Bellido M, Lugo L, Roman-Blas JA, et al. Improving subchondral bone integrity reduces progression of cartilage damage in experimental osteoarthritis preceded by osteoporosis. Osteoarthritis Cartilage. 2011;19(10): 1228-1236.[31] Zeng JZ, Wang ZZ, Ma LF, et al. Increased receptor activator of nuclear factor kappabeta ligand/osteoprotegerin ratio exacerbates cartilage destruction in osteoarthritis in vitro. Exp Ther Med. 2016;12(4):2778-2782.[32] Sagar DR, Ashraf S, Xu L, et al. Osteoprotegerin reduces the development of pain behaviour and joint pathology in a model of osteoarthritis. Ann Rheum Dis. 2014;73(8):1558-1565.[33] Abed E, Bouvard B, Martineau X, et al. Elevated hepatocyte growth factor levels in osteoarthritis osteoblasts contribute to their altered response to bone morphogenetic protein-2 and reduced mineralization capacity. Bone. 2015;75:111-119.[34] Shibasaki S, Tsunemi S, Kitano S, et al. Differential regulation of c-Met signaling pathways for synovial cell function. Springerplus. 2014;3:554.[35] Oh H, Kwak JS, Yang S, et al. Reciprocal regulation by hypoxia-inducible factor-2alpha and the NAMPT-NAD(+)-SIRT axis in articular chondrocytes is involved in osteoarthritis. Osteoarthritis Cartilage. 2015; 23(12):2288-2296.[36] Bae WJ, Shin MR, Kang SK, et al. HIF-2 Inhibition Supresses Inflammatory Responses and Osteoclastic Differentiation in Human Periodontal Ligament Cells. J Cell Biochem. 2015; 116(7):1241-1255.[37] Lin N Y, Distler A, Beyer C, et al. Inhibition of Notch1 promotes hedgehog signalling in a HES1-dependent manner in chondrocytes and exacerbates experimental osteoarthritis. Ann Rheum Dis. 2016;75(11):2037-2044.[38] Ryu J H, Shin Y, Huh Y H, et al. Hypoxia-inducible factor-2alpha regulates Fas-mediated chondrocyte apoptosis during osteoarthritic cartilage destruction. Cell Death Differ. 2012;19(3):440-450.[39] Saito T, Fukai A, Mabuchi A, et al. Transcriptional regulation of endochondral ossification by HIF-2 alpha during skeletal growth and osteoarthritis development. Nat Med. 2010; 16(6):678-686.[40] Ishizuka S, Sakai T, Hiraiwa H, et al. Hypoxia-inducible factor-2alpha induces expression of type X collagen and matrix metalloproteinases 13 in osteoarthritic meniscal cells. Inflamm Res. 2016;65(6):439-448.[41] Markway BD, Cho H, Zilberman-Rudenko J, et al. Hypoxia-inducible factor 3-alpha expression is associated with the stable chondrocyte phenotype. J Orthop Res. 2015;33(11):1561-1570.[42] van der Kraan PM, Blaney DE, van den Berg WB. A role for age-related changes in TGFbeta signaling in aberrant chondrocyte differentiation and osteoarthritis. Arthritis Res Ther. 2010;12(1):201.[43] Zhen G, Wen C, Jia X, et al. Inhibition of TGF-beta signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med. 2013;19(6):704-712.[44] Lu C, Wan Y, Cao J, et al. Wnt-mediated reciprocal regulation between cartilage and bone development during endochondral ossification. Bone. 2013;53(2):566-574.[45] Kuchler U, Schwarze U Y, Dobsak T, et al. Dental and periodontal phenotype in sclerostin knockout mice. Int J Oral Sci. 2014;6(2):70-76.[46] Weng LH, Wang CJ, Ko J Y, et al. Control of Dkk-1 ameliorates chondrocyte apoptosis, cartilage destruction, and subchondral bone deterioration in osteoarthritic knees. Arthritis Rheum. 2010;62(5):1393-1402.[47] Inkson CA, Ono M, Kuznetsov S A, et al. TGF-beta1 and WISP-1/CCN-4 can regulate each other's activity to cooperatively control osteoblast function. J Cell Biochem. 2008;104(5):1865-1878.[48] Walsh DA, Mcwilliams DF, Turley MJ, et al. Angiogenesis and nerve growth factor at the osteochondral junction in rheumatoid arthritis and osteoarthritis. Rheumatology (Oxford). 2010;49(10):1852-1861.[49] Nagai T, Sato M, Kutsuna T, et al. Intravenous administration of anti-vascular endothelial growth factor humanized monoclonal antibody bevacizumab improves articular cartilage repair. Arthritis Res Ther. 2010;12(5):R178.[50] Lane NE, Schnitzer TJ, Birbara CA, et al. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med. 2010;363(16):1521-1531.[51] Balanescu AR, Feist E, Wolfram G, et al. Efficacy and safety of tanezumab added on to diclofenac sustained release in patients with knee or hip osteoarthritis: a double-blind, placebo-controlled, parallel-group, multicentre phase III randomised clinical trial. Ann Rheum Dis. 2014;73(9): 1665-1672.[52] Brown MT, Murphy FT, Radin DM, et al. Tanezumab reduces osteoarthritic hip pain: results of a randomized, double-blind, placebo-controlled phase III trial. Arthritis Rheum. 2013; 65(7):1795-1803.[53] Spierings EL, Fidelholtz J, Wolfram G, et al. A phase III placebo- and oxycodone-controlled study of tanezumab in adults with osteoarthritis pain of the hip or knee. Pain. 2013; 154(9):1603-1612.[54] 王呈,薄其玉,戴国锋,等.小分子药物Kartogenin诱导骨髓间充质干细胞定向分化中基质金属蛋白酶2的表达[J].中国组织工程研究,2016,20(50):7475-7480. |
| [1] | Zhang Jichao, Dong Yuefu, Mou Zhifang, Zhang Zhen, Li Bingyan, Xu Xiangjun, Li Jiayi, Ren Meng, Dong Wanpeng. Finite element analysis of biomechanical changes in the osteoarthritis knee joint in different gait flexion angles [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1357-1361. |
| [2] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [3] | Jin Tao, Liu Lin, Zhu Xiaoyan, Shi Yucong, Niu Jianxiong, Zhang Tongtong, Wu Shujin, Yang Qingshan. Osteoarthritis and mitochondrial abnormalities [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1452-1458. |
| [4] | Wang Baojuan, Zheng Shuguang, Zhang Qi, Li Tianyang. Miao medicine fumigation can delay extracellular matrix destruction in a rabbit model of knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1180-1186. |
| [5] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [6] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [7] | Liu Dongcheng, Zhao Jijun, Zhou Zihong, Wu Zhaofeng, Yu Yinghao, Chen Yuhao, Feng Dehong. Comparison of different reference methods for force line correction in open wedge high tibial osteotomy [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(6): 827-831. |
| [8] | Zhou Jianguo, Liu Shiwei, Yuan Changhong, Bi Shengrong, Yang Guoping, Hu Weiquan, Liu Hui, Qian Rui. Total knee arthroplasty with posterior cruciate ligament retaining prosthesis in the treatment of knee osteoarthritis with knee valgus deformity [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(6): 892-897. |
| [9] | Xu Lei, Han Xiaoqiang, Zhang Jintao, Sun Haibiao. Hyaluronic acid around articular chondrocytes: production, transformation and function characteristics [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 768-773. |
| [10] | He Junjun, Huang Zeling, Hong Zhenqiang. Interventional effect of Yanghe Decoction on synovial inflammation in a rabbit model of early knee osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 694-699. |
| [11] | Lin Xuchen, Zhu Hainian, Wang Zengshun, Qi Tengmin, Liu Limin, Suonan Angxiu. Effect of xanthohumol on inflammatory factors and articular cartilage in a mouse mode of osteoarthritis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(5): 676-681. |
| [12] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [13] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [14] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| [15] | Zhang Tong, Cai Jinchi, Yuan Zhifa, Zhao Haiyan, Han Xingwen, Wang Wenji. Hyaluronic acid-based composite hydrogel in cartilage injury caused by osteoarthritis: application and mechanism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 617-625. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||