Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (17): 2714-2721.doi: 10.3969/j.issn.2095-4344.2017.17.015
Previous Articles Next Articles
Comparison of three kinds of mesenchymal stem cells differentiating into nerve cells under co-culture induction
Xu Li-li1, Wang Hong-yuan2, Li Xue-da3, Liu Bing4, Zheng Fang-fang1, Yang Nai-long1
- 1Department of Endocrinology and Metabolic Disease, 2Comprehesive Department, 3Interventional Center, 4Department of Vascular Surgery, Qingdao University Affiliated Hospital, Qingdao 266000, Shandong Province, China
-
Revised:2017-02-16Online:2017-06-18Published:2017-06-29 -
Contact:Yang Nai-long, Master, Chief physician, Department of Endocrinology and Metabolic Disease, Qingdao University Affiliated Hospital, Qingdao 266000, Shandong Province, China -
About author:Xu Li-li, Master, Attending physician, Department of Endocrinology and Metabolic Disease, Qingdao University Affiliated Hospital, Qingdao 266000, Shandong Province, China Liu Bing, M.D., Attending physician, Department of Vascular Surgery, Qingdao University Affiliated Hospital, Qingdao 266000, Shandong Province, China Xu Li-li and Liu Bing contributed equally to this work. -
Supported by:the Medical and Health Science Development Plan of Shandong Province in 2009, No. HW027
CLC Number:
Cite this article
Xu Li-li, Wang Hong-yuan, Li Xue-da, Liu Bing, Zheng Fang-fang, Yang Nai-long. Comparison of three kinds of mesenchymal stem cells differentiating into nerve cells under co-culture induction[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(17): 2714-2721.
share this article
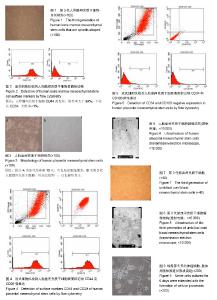
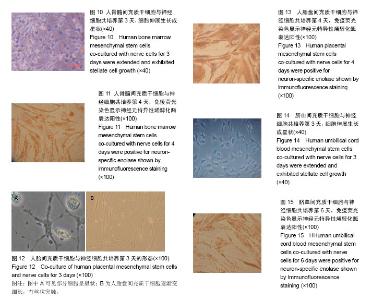
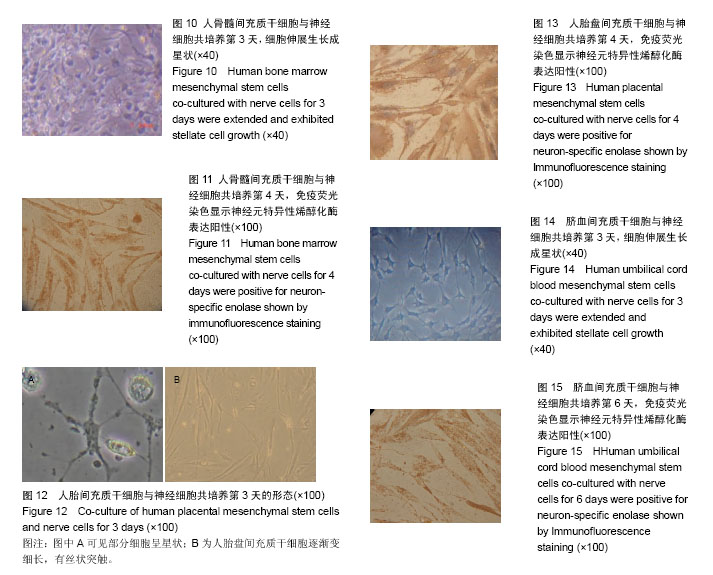
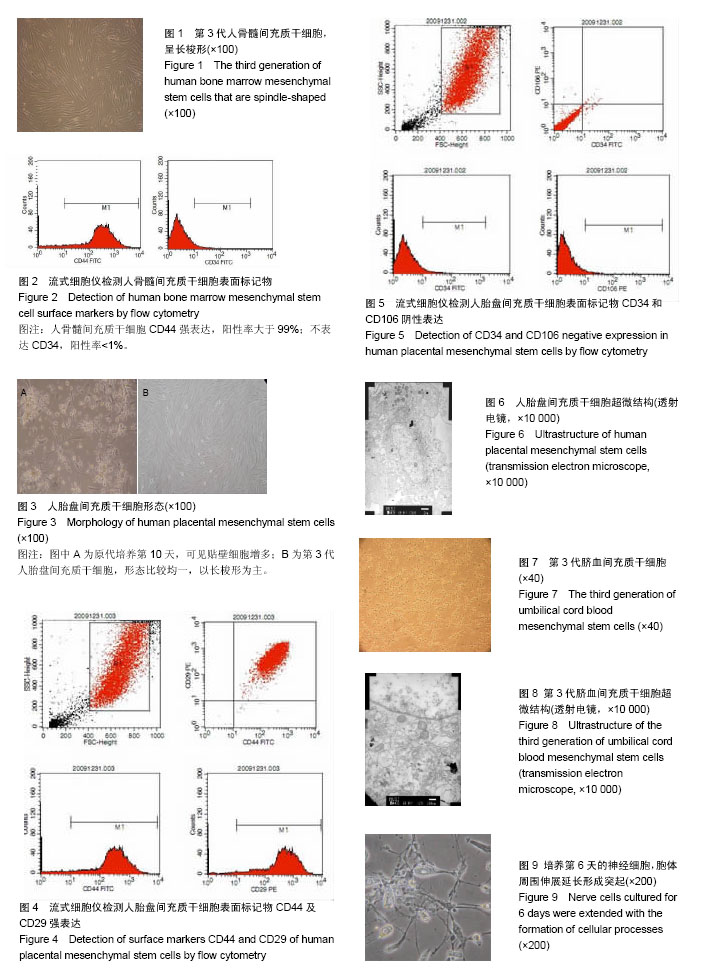
2.1 人骨髓间充质干细胞形态 细胞在第2天时开始贴壁生长,细胞两端有较长的突起,细胞核呈卵圆形,细胞克隆样生长,集落大小不一,细胞刚开始生长较缓慢,2周时融合可达85%左右。传代之后细胞生长均匀分布,为长梭形形态(图1),有两三天的倍增时间。经过反复传代,其纯度可超过90%。 2.2 人骨髓间充质干细胞表面标志物表达 通过流式细胞仪检测,人骨髓间充质干细胞CD44强表达,阳性率大于99%;不表达CD34,阳性率<1% (图2)。 2.3 人胎盘间充质干细胞的形态 在第7天可见少量的细胞贴壁生长,第10天可见贴壁细胞增多(图3A),第18天可见细胞融合约75%,细胞呈梭形、圆形和不规则形等形态,并混杂其他细胞杂质,传代之后细胞生长速度显著增快,三四天可传1代。传代之后细胞形态比较均一,以长梭形、平行生长为主,也有细胞表现为火焰、漩涡状生长,第3代之后细胞的纯度显著增高(图3B)。 2.4 人胎盘间充质干细胞表面标志物表达 通过流式细胞仪检测,人胎盘间充质干细胞CD44及CD29强表达,两者阳性率均大于99%;CD34、CD106不表达,阳性率均< 1%(图4,5)。 2.5 人胎盘间充质干细胞超微结构 通过透射电镜可看到细胞为长梭形,核仁较明显,核异染色质比较少,胞浆中有扩张的微管、微丝以及粗面内质网,且可看到内质网分泌微丝;少数细胞的核质比较大,胞浆较少,胞浆中的细胞器较少,符合间充质干细胞的特点(图6)。 2.6 脐血间充质干细胞形态 细胞在第2天开始贴壁生长,细胞核呈卵圆形,细胞两端有较长的突起,克隆样生长,集落形成大小不一,细胞一开始生长较慢,2周后融合可达85%左右。细胞的形态呈多样化,如星形、小圆形、不规则形等。形态与人骨髓间充质干细胞相似,但体积较小,随着培养时间的延长,细胞的体积会逐渐变大并伸展生长(图7)。 2.7 脐血间充质干细胞表面标志物表达 通过流式细胞仪检测,脐血间充质干细胞CD44及CD29均呈强表达;CD34不表达,符合间充质干细胞的特征。 2.8 脐血间充质干细胞超微结构 通过透射电镜可见脐血间充质干细胞的细胞核呈类圆形以及不规则形,核膜清晰,核仁明显,染色质较粗且位于核膜周围。细胞质中细胞器的种类及数量众多,如微管、微丝、线粒体、粗面内质网等。通过扫描电镜可以看到在盖玻片表面贴壁生长的间充质干细胞,细胞呈长梭形,突起较长,可在细胞的表面看见微绒毛(图8)。 2.9 神经细胞形态 培养第2天,大部分神经细胞已经贴壁,胞体形态多呈纺锤形,伸出小的突起。有些细胞的胞体形态呈体积较大的扁平形,并伸出数量不等的棒状大突起,表现为胶质细胞。培养6 d后,神经细胞胞体变大,细胞呈圆形及椭圆形,胞体周围伸展延长而成为单极、双极或者多极的突起(图9)。神经元特异性烯醇化酶染色阳性。 2.10 共培养细胞形态变化 2.10.1 人骨髓间充质干细胞与神经细胞共培养结果 共培养体系中,在第3天即可观察到人骨髓间充质干细胞的形态变化,扁平的细胞缩小,胞体多呈圆形并形成较多突起,细胞形态逐渐成为星状(图10)。第4天之后星状细胞变多,并且可以互相连接,形态具有神经样特征,免疫荧光染色显示神经元特异性烯醇化酶表达阳性(图11),阳性率平均为(32.7±11.5)%,表现为神经样细胞的特征;对照组培养约8 d后,大部分人骨髓间充质干细胞的形态仍旧扁而宽,没有形成神经样形态结构,免疫荧光染色显示神经元特异性烯醇化酶表达阴性。 2.10.2 人胎盘间充质干细胞与神经细胞共培养结果 共培养体系中,在第3天即可观察到人胎盘间充质干细胞逐渐由之前的扁平变得细长,且可以看见丝状突触,部分细胞呈星状(图12),对照组人胎盘间充质干细胞没有显著的改变。共培养4 d后免疫荧光染色显示神经元特异性烯醇化酶表达阳性(图13),阳性率平均(35.3±13.5)%,表现出神经样细胞特性。 2.10.3 脐血间充质干细胞与神经细胞共培养结果 共培养体系中,在第3天可见脐血间充质干细胞由之前的扁平形逐渐缩小,胞体多数呈圆形,并且形成了突起,部分细胞形态呈星状或者网格状(图14)。第6天之后星状细胞逐渐变多并互相连接,表现出神经样细胞的特征,免疫荧光染色显示神经元特异性烯醇化酶表达阳性(图15),阳性率平均(37.5±12.7)%,具有神经样细胞的特性;而对照组脐血间充质干细胞培养第8天时,大多数细胞仍是长梭样形态,没有形成神经样的形态结构,免疫荧光染色显示神经元特异性烯醇化酶表达阴性。"
| [1] Yan X, Xu N, Meng C, et al. Generation of induced pluripotent stem cells from human mesenchymal stem cells of parotid gland origin. Am J Transl Res. 2016;8(2):419-432.[2] Li X, Bai J, Ji X, et al. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int J Mol Med. 2014;34(3):695-704.[3] Mouiseddine M, François S, Semont A, et al. Human mesenchymal stem cells home specifically to radiation-injured tissues in a non-obese diabetes/severe combined immunodeficiency mouse model. Br J Radiol. 2007;80 Spec No 1:S49-55.[4] Cordonnier T, Langonné A, Corre P, et al. Osteoblastic differentiation and potent osteogenicity of three-dimensional hBMSC-BCP particle constructs. J Tissue Eng Regen Med. 2014;8(5):364-376.[5] Alfred R, Taiani JT, Krawetz RJ, et al. Large-scale production of murine embryonic stem cell-derived osteoblasts and chondrocytes on microcarriers in serum-free media. Biomaterials. 2011;32(26):6006-6016.[6] Sanchez-Ramos J, Song S, Cardozo-Pelaez F, et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164(2):247-256.[7] Woodbury D, Reynolds K, Black IB. Adult bone marrow stromal stem cells express germline, ectodermal, endodermal, and mesodermal genes prior to neurogenesis. J Neurosci Res. 2002;69(6):908-917.[8] Woodbury D, Schwarz EJ, Prockop DJ, et al. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61(4):364-370.[9] Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999;96(19):10711-10716.[10] Yan X, Xu N, Meng C, et al. Generation of induced pluripotent stem cells from human mesenchymal stem cells of parotid gland origin. Am J Transl Res. 2016;8(2):419-432.[11] Zhang H, Huang Z, Xu Y, et al. Differentiation and neurological benefit of the mesenchymal stem cells transplanted into the rat brain following intracerebral hemorrhage. Neurol Res. 2006;28(1):104-112.[12] Marappagounder D, Somasundaram I, Dorairaj S, et al. Differentiation of mesenchymal stem cells derived from human bone marrow and subcutaneous adipose tissue into pancreatic islet-like clusters in vitro. Cell Mol Biol Lett. 2013; 18(1):75-88.[13] Tasev D, Konijnenberg LS, Amado-Azevedo J, et al. CD34 expression modulates tube-forming capacity and barrier properties of peripheral blood-derived endothelial colony- forming cells (ECFCs). Angiogenesis. 2016;19(3):325-338.[14] Zhang H, Yu N, Zhou Y, et al. Construction and characterization of osteogenic and vascular endothelial cell sheets from rat adipose-derived mesenchymal stem cells. Tissue Cell. 2016;48(5):488-495.[15] Liang G, Li S, Du W,et al. Hypoxia regulates CD44 expression via hypoxia-inducible factor-1α in human gastric cancer cells. Oncology Letters. 2017;13:967-972.[16] Abreu Velez AM, Upegui Zapata YA, Howard MS. Periodic Acid-Schiff Staining Parallels the Immunoreactivity Seen By Direct Immunofluorescence in Autoimmune Skin Diseases. N Am J Med Sci. 2016;8(3):151-155.[17] Asadi A, Bruin JE, Kieffer TJ. Characterization of Antibodies to Products of Proinsulin Processing Using Immunofluorescence Staining of Pancreas in Multiple Species. J Histochem Cytochem. 2015;63(8):646-662.[18] Mannoji C, Koda M, Kamiya K, et al. Transplantation of human bone marrow stromal cell-derived neuroregenrative cells promotes functional recovery after spinal cord injury in mice. Acta Neurobiol Exp (Wars). 2014;74(4):479-488.[19] Fedyanin M, Anna P, Elizaveta P, et al. Role of Stem Cells in Colorectal Cancer Progression and Prognostic and Predictive Characteristics of Stem Cell Markers in Colorectal Cancer. Curr Stem Cell Res Ther. 2017;12(1):19-30.[20] Lee M, Jeong SY, Ha J, et al. Low immunogenicity of allogeneic human umbilical cord blood-derived mesenchymal stem cells in vitro and in vivo. Biochem Biophys Res Commun. 2014;446(4):983-989.[21] 王志勇,王永安,熊敏,等.脑红蛋白基因修饰的骨髓间充质干细胞在治疗大鼠脊髓损伤的保护作用[J].中华实验外科杂志,2015, 32(6):1378-1380.[22] 王昌铭,滕军放,高唱,等.骨髓间充质干细胞颈内动脉移植在血管性痴呆大鼠脑内存活迁移及对胆碱能系统的影响[J].中华老年心脑血管病杂志,2014,16(2):196-199.[23] 陈玉玺,王晓莉,牟青杰,等.骨髓间充质干细胞对脑缺血大鼠星形胶质细胞影响的实验研究[J].医学研究生学报,2013,26(6):564-567.[24] 刘明,夏玉军,张明,等.三维球体间充质干细胞移植对大鼠缺血再灌注损伤脑组织Nogo-A及NgR表达的影响[J].第二军医大学学报, 2015,36(10):1087-1091.[25] 丁红芳,丁慧芳,高欣义,等.胎盘间充质干细胞治疗大鼠缺氧缺血性脑病的实验研究[J].实用医学杂志,2013,29(5): 711-714.[26] Haque A, Ray SK, Cox A, et al. Neuron specific enolase: a promising therapeutic target in acute spinal cord injury. Metab Brain Dis. 2016;31(3):487-495.[27] 赵俊暕,陈乃耀,石峻,等.人脐血间充质干细胞移植对创伤性脑损伤大鼠VEGF分泌及血管新生的影响[J].中国神经免疫学和神经病学杂志,2013,20(4):267-273.[28] 陈镭,惠国桢,张赛,等.人脐血间充质干细胞移植促进大鼠颅脑创伤后神经功能的恢复[J].中华创伤杂志,2009,25(6):498-502.[29] 颜小华,黄瑞滨.脐血间充质干细胞静脉移植治疗新生大鼠缺氧缺血性脑损伤的时效性研究[J].中国小儿急救医学,2006,13(4): 322-324.[30] Sherief LM, Beshir M, Salah HE. Neuron-specific enolase in cerebrospinal fluid as a neurochemical marker for brain damage in acute lymphoblastic leukemia. Egypt J Haematol. 2016;41(2):77-80.[31] Bao X, Ren T, Huang Y, et al. Bortezomib induces apoptosis and suppresses cell growth and metastasis by inactivation of Stat3 signaling in chondrosarcoma. Int J Oncol. 2017;50(2): 477-486.[32] Musa ZA, Qasim BJ, Ghazi HF, et al. Diagnostic roles of calretinin in hirschsprung disease: A comparison to neuron-specific enolase. Saudi J Gastroenterol. 2017;23(1): 60-66.[33] Zhang Y, Schedle A, Matejka M, et al. The proliferation and differentiation of osteoblasts in co-culture with human umbilical vein endothelial cells: An improved analysis using fluorescence-activated cell sorting. Cell Mol Biol Lett. 2010; 15(4):517-529.[34] Liu Y, Shao LL, Pang W, et al. Induction of adhesion molecule expression in co-culture of human bronchial epithelial cells and neutrophils suppressed by puerarin via down-regulating p38 mitogen-activated protein kinase and nuclear factor κB pathways. Chin J Integr Med. 2014;20(5):360-368.[35] Xiong L, Huang C, Chen XF, et al. Comparison of fermentation by mono-culture and co-culture of oleaginous yeasts for ABE (acetone- butanol- ethanol) fermentation wastewater treatment. Journal of Environmental Chemical Engineering. 2016;4(4):3803-3809.[36] Zhao S, Yuan L, Wang J, et al. A novel and facile approach to imaging nanoparticles transport across Transwell filter grown cell monolayer in real-time and in situ under confocal laser scanning microscopy. Biol Pharm Bull. 2012;35(3):335-345.[37] Wu Y, Li K, Zuo H, et al. Primary neuronal-astrocytic co-culture platform for neurotoxicity assessment of di-(2-ethylhexyl) phthalate. J Environ Sci (China). 2014; 26(5):1145-1153.[38] Yang X, Yan H, Zhai Z, et al. Neutrophil elastase promotes proliferation of HaCaT cell line and transwell psoriasis organ culture model. Int J Dermatol. 2010;49(9):1068-1074.[39] 谢婷婷,杨乃龙,徐丽丽,等.尿酸影响人骨髓间充质干细胞成骨分化过程中BMP-2表达[J].现代生物医学进展, 2015,15(12): 2251-2256.[40] 徐丽丽,孙晓娟,郝秀仙,等.在复合成分培养基中骨髓间充质干细胞的诱导成骨[J].中国组织工程研究, 2015,19(10):1501-1505.[41] 邵帅,周晨红,徐丽丽.体外诱导骨髓与脐血间充质干细胞向成骨细胞分化及其成骨活性的比较[J].中国组织工程研究,2015,19 (23):3652-3657.[42] 王潇丽,徐丽丽,杨乃龙.尿酸对人骨髓间充质干细胞成骨分化过程中Wnt信号通路的影响[J].中国组织工程研究,2015, 19(28): 4472-4477.[43] Liu Y, Wang Y, Yang N, et al. In silico analysis of the molecular mechanism of postmenopausal osteoporosis. Mol Med Rep. 2015;12(5):6584-6590.[44] Han F, Guo Y, Xu L, et al. Induction of Haemeoxygenase-1 Directly Improves Endothelial Function in Isolated Aortas from Obese Rats through the Ampk-Pi3k/Akt-Enos Pathway. Cell Physiol Biochem. 2015;36(4):1480-1490.[45] 李颖,王潇丽,王军,等. GLP-1受体激动剂艾塞那肽对MG-63细胞增殖及成骨分化的影响[J].中国骨质疏松杂志,2016,22(4): 50-53.[46] Li JJ, Li Y, Wang J, et al.Correlation between vitamin D levels and static postural balance of both feet in type 2 diabetes mellitus patients. Int J Clin Exp Med .2016;9(6):11651-11656. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [3] | Xiao Hao, Liu Jing, Zhou Jun. Research progress of pulsed electromagnetic field in the treatment of postmenopausal osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1266-1271. |
| [4] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [5] | Hou Jingying, Guo Tianzhu, Yu Menglei, Long Huibao, Wu Hao. Hypoxia preconditioning targets and downregulates miR-195 and promotes bone marrow mesenchymal stem cell survival and pro-angiogenic potential by activating MALAT1 [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1005-1011. |
| [6] | Liang Xuezhen, Yang Xi, Li Jiacheng, Luo Di, Xu Bo, Li Gang. Bushen Huoxue capsule regulates osteogenic and adipogenic differentiation of rat bone marrow mesenchymal stem cells via Hedgehog signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1020-1026. |
| [7] | Wen Dandan, Li Qiang, Shen Caiqi, Ji Zhe, Jin Peisheng. Nocardia rubra cell wall skeleton for extemal use improves the viability of adipogenic mesenchymal stem cells and promotes diabetes wound repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1038-1044. |
| [8] | Zhu Bingbing, Deng Jianghua, Chen Jingjing, Mu Xiaoling. Interleukin-8 receptor enhances the migration and adhesion of umbilical cord mesenchymal stem cells to injured endothelium [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1045-1050. |
| [9] | Fang Xiaolei, Leng Jun, Zhang Chen, Liu Huimin, Guo Wen. Systematic evaluation of different therapeutic effects of mesenchymal stem cell transplantation in the treatment of ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1085-1092. |
| [10] | Guo Jia, Ding Qionghua, Liu Ze, Lü Siyi, Zhou Quancheng, Gao Yuhua, Bai Chunyu. Biological characteristics and immunoregulation of exosomes derived from mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1093-1101. |
| [11] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [12] | Tian Chuan, Zhu Xiangqing, Yang Zailing, Yan Donghai, Li Ye, Wang Yanying, Yang Yukun, He Jie, Lü Guanke, Cai Xuemin, Shu Liping, He Zhixu, Pan Xinghua. Bone marrow mesenchymal stem cells regulate ovarian aging in macaques [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 985-991. |
| [13] | Chen Xiaoxu, Luo Yaxin, Bi Haoran, Yang Kun. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 591-596. |
| [14] | Kang Kunlong, Wang Xintao. Research hotspot of biological scaffold materials promoting osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 597-603. |
| [15] | Shen Jiahua, Fu Yong. Application of graphene-based nanomaterials in stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 604-609. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||