Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (17): 2644-2651.doi: 10.3969/j.issn.2095-4344.2017.17.004
Previous Articles Next Articles
Optimization of Raman spectra acquisition conditions and its application in a comparison study on mesenchymal stem cells and embryonic stem cells
Chen Ming, Cheng Xue-lian, Duan Yong-juan, Hu Xiao
- State Key Laboratory of Experimental Hematology, Institute of Hematology & Hospital of Blood Diseases, Chinese Academy of Medical Sciences & Peking Union Medical College, Tianjin 300020, China
-
Revised:2017-05-05Online:2017-06-18Published:2017-06-29 -
Contact:Hu Xiao, M.D., Researcher, State Key Laboratory of Experimental Hematology, Institute of Hematology & Hospital of Blood Diseases, Chinese Academy of Medical Sciences & Peking Union Medical College, Tianjin 300020, China -
About author:Chen Ming, Master, State Key Laboratory of Experimental Hematology, Institute of Hematology & Hospital of Blood Diseases, Chinese Academy of Medical Sciences & Peking Union Medical College, Tianjin 300020, China -
Supported by:the National High-Technology Research and Development Program of China (863 Program), No. 2011AA020118; the National Basic Research Program of China (973 Program), No. 2012CB966504
CLC Number:
Cite this article
Chen Ming, Cheng Xue-lian, Duan Yong-juan, Hu Xiao. Optimization of Raman spectra acquisition conditions and its application in a comparison study on mesenchymal stem cells and embryonic stem cells[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(17): 2644-2651.
share this article
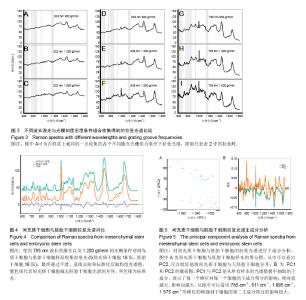
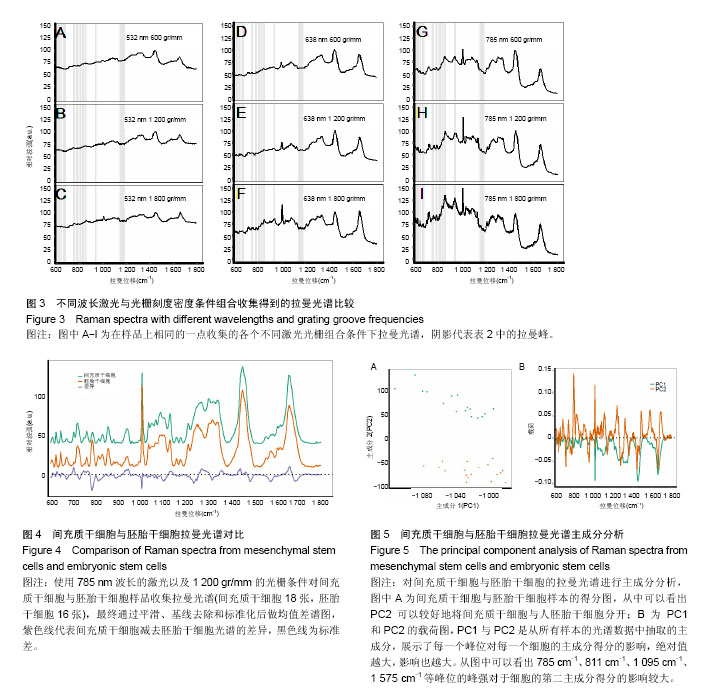
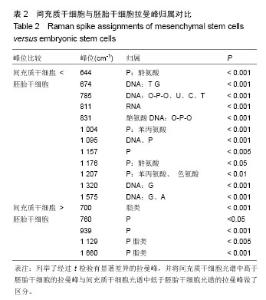
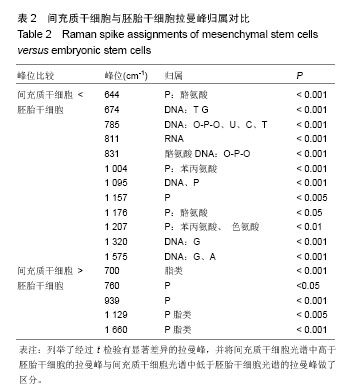
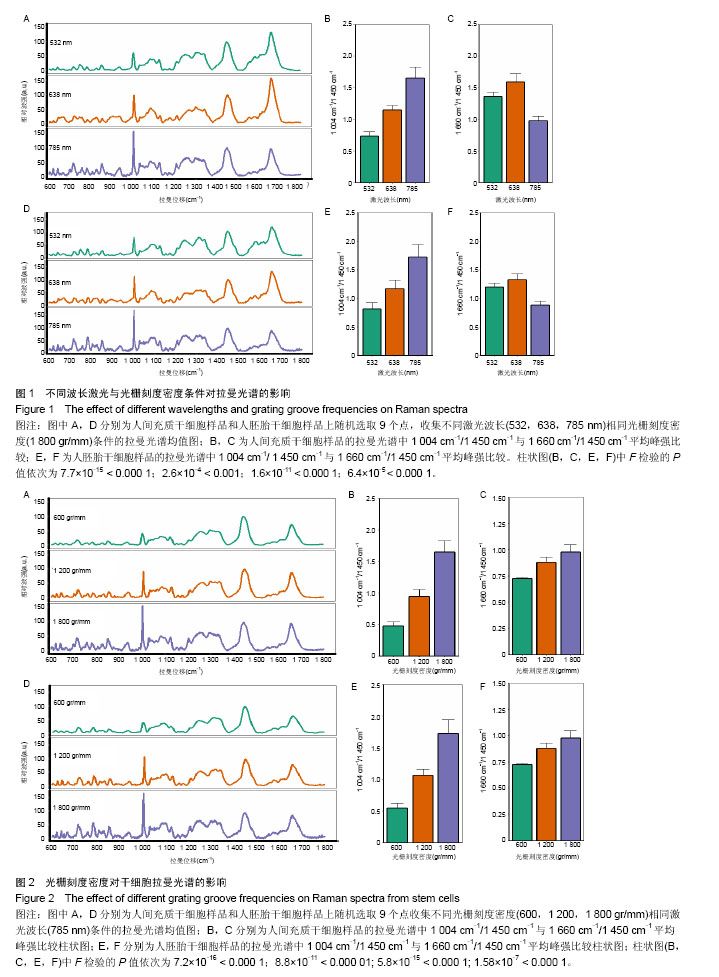
2.1 激光波长对人间充质干细胞和人胚胎干细胞拉曼光谱中拉曼峰的影响 为了验证激光波长对干细胞拉曼光谱的影响,首先比较在不同波长的激光(532,638,785 nm)下人间充质干细胞(图1A-C)与人胚胎干细胞(图1D-F)的拉曼光谱。 从图1A与图1D中可以看出3种不同条件下的光谱有多处不同,785 nm条件下光谱在600 cm-1到1 000 cm-1之间具有更清晰的峰型,而532 nm条件下光谱在1 500 cm-1到 1 700 cm-1之间有较高隆起。人间充质干细胞拉曼光谱中1 004 cm-1的平均峰强在3种不同波长激光下依次为基准峰1 450 cm-1的0.74倍、1.15倍和1.65倍(图1B),人胚胎干细胞拉曼光谱中1 004 cm-1的平均峰强在3种不同波长激光下依次为基准峰1 450 cm-1的0.82倍、1.17倍和1.71倍(图1E);而人间充质干细胞拉曼光谱中1 660 cm-1的平均峰强相比较于基准峰则依次为1.36倍、1.59倍、0.98倍(图1C),人胚胎干细胞拉曼光谱中1 660 cm-1的平均峰强相比较于基准峰则依次为1.19倍、1.32倍和0.87倍(图1F)。1 004 cm-1/1 450 cm-1在785 nm波长的激光下比值最大,而1 660 cm-1/1 450 cm-1则在638 nm波长的激光下比值最大。 2.2 光栅刻度密度对人间充质干细胞和人胚胎干细胞拉曼光谱的影响 为了验证光栅刻度密度对于干细胞拉曼光谱的影响,进一步比较不同光栅刻度密度(600,1 200, 1 800 gr/mm)条件下人间充质干细胞(图2A-C)和人胚胎干细胞(图2D-F)拉曼光谱的差异。 从图2A和图2D中可以看出1 800 gr/mm条件下的光谱在600 cm-1到1 000 cm-1之间相比于1 200 gr/mm和600 gr/mm条件下得到的拉曼光谱具有更加清晰的峰。同时人间充质干细胞拉曼光谱中1 004 cm-1的峰强依次是基准峰1 450 cm-1的0.48倍、0.95倍和1.64倍(图2B),而1 660 cm-1的峰强则是基准峰1 450 cm-1的0.72倍、0.88倍和0.98倍(图2C);人胚胎干细胞拉曼光谱中1 004 cm-1的峰强依次是基准峰1 450 cm-1的0.54倍、1.06倍和1.71倍(图2E),1 660 cm-1的峰强则是基准峰1450 cm-1的0.64倍、0.76倍和0.87倍(图2F)。1 004 cm-1/ 1450 cm-1的均值无论是在人间充质干细胞还是人胚胎干细胞样品中其最大值都出现在1 800 gr/mm的光栅刻度密度条件下,而最小值则出现在600 gr/mm的光栅刻度密度条件下。随着光栅刻度线密度的增加,1 004 cm-1/1 450 cm-1和1 660 cm-1/1 450 cm-1的比值都是逐渐增加的,但是1 004 cm-1/1 450 cm-1的比值增加程度相比于1 660 cm-1/1 450 cm-1的增加程度更明显。 2.3 最佳激光波长和光栅密度光谱收集条件组合的确定 在上述工作的基础上,进一步根据光谱分辨率与光谱总收集时间从3种激光波长与3种光栅刻度密度条件组合的9种不同条件(图3A-I)中筛选最佳的一个组合。根据已发表文献中人间充质干细胞的拉曼光谱的重要拉曼峰(在图中用阴影部分区分)在图中是否能较好识别以及组成光谱的数据点数的多少来选择光谱分辨率较高的光谱(表1)[16,23]。 从图3A-I中可以看出,光谱在阴影部分的峰位上逐渐变得清晰和突出,其中A-E在阴影部分标注出大部分的峰位表现较为平滑甚至难以识别。根据表1可知只有图3F(638 nm 1 800 gr/mm)、图3H(785 nm,1 200 gr/mm)、图3I(785 nm,1 800 gr/mm)是由超过1 000个数据点构成,这3种光谱相较于其他的光谱在阴影部分具有更加清晰的拉曼峰位,这3种光谱的收集时间分别为100×4 s、100×4 s和100×8 s。 2.4 利用优化的组合条件比较间充质干细胞与胚胎干细胞拉曼光谱 胚胎干细胞与间充质干细胞同为重要的干细胞,分别是全能干细胞与多能干细胞,在组织工程上均可作为种子细胞,通过拉曼光谱可以寻找这2种干细胞的内在特性与区别,为干细胞的进一步研究与应用奠定基础。根据上述研究获得的最佳拉曼光谱收集条件 785 nm和1 200 gr/mm,分别收集间充质干细胞与胚胎干细胞的拉曼光谱图,并对结果求均值后得到如图4的差谱图。从图中可以看出,间充质干细胞与胚胎干细胞拉曼光谱的差异主要体现在785 cm-1、811 cm-1、1 095 cm-1、1 575 cm-1等拉曼峰。 表2列举了间充质干细胞与胚胎干细胞有显著差异的拉曼光谱峰位,并将人间充质干细胞更强的峰和人胚胎干细胞更强的峰做了区分。 2.5 间充质干细胞与胚胎干细胞拉曼光谱的主成分分析 由于间充质干细胞与胚胎干细胞之间在多个波数位置有差异,为了找出这2种干细胞的特征性差异,较好的区分这2种干细胞,为未来拉曼光谱应用于组织工程和干细胞检测提供理论基础,实验借助多元统计学分析方法主成分分析来区分间充质干细胞与胚胎干细胞的拉曼光谱。主成分分析通过降维的思想从众多差异峰位抽提出2个主成分PC1(主成分1)和PC2(主成分2)来代替这些差异,每一张光谱都具有一个PC1和PC2的得分,据此可以将不同的光谱区分开。 图5A通过主成分分析抽取出2个主成分PC1(98.6%)和PC2(0.5%)来代替间充质干细胞与胚胎干细胞拉曼光谱多个峰位的差异,从而简化间充质干细胞与胚胎干细胞的区分问题。可以看出利用抽取的前2个主成分可以很好地将间充质干细胞与胚胎干细胞的拉曼光谱区分开,同时,通过图5B的载荷图可以看出影响PC2的主要差异就在 785 cm-1、811 cm-1、1 095 cm-1、1 575 cm-1等峰位。"
| [1] Bernstein HS, Srivastava D. Stem cell therapy for cardiac disease. Pediatr Res. 2012;71(4 Pt 2):491-499.[2] Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451(7181):937-942.[3] Vrtovec B, Sever M, Jensterle M, et al. Efficacy of CD34+ Stem Cell Therapy in Nonischemic Dilated Cardiomyopathy Is Absent in Patients With Diabetes but Preserved in Patients With Insulin Resistance. Stem Cells Transl Med. 2016;5(5): 632-638.[4] Aksoy C, Severcan F,. Role of vibrational spectroscopy in stem cell research. Spectroscopy. 2012;27(27):167-184.[5] 荣曦,黄庶识,刘红.拉曼光谱分析技术在细胞生物学研究中的应用进展[J].激光生物学报,2010,19(1):136-142.[6] Ilin Y, Kraft ML. Secondary ion mass spectrometry and Raman spectroscopy for tissue engineering applications. Curr Opin Biotechnol. 2015;31:108-116.[7] Konorov SO, Schulze HG, Piret JM, et al. Label-free determination of the cell cycle phase in human embryonic stem cells by Raman microspectroscopy. Anal Chem. 2013; 85(19):8996-9002.[8] Hofemeier AD, Hachmeister H, Pilger C, et al. Label-free nonlinear optical microscopy detects early markers for osteogenic differentiation of human stem cells. Sci Rep. 2016;6:26716.[9] Mitchell A, Ashton L, Yang XB, et al. Detection of early stage changes associated with adipogenesis using Raman spectroscopy under aseptic conditions. Cytometry A. 2015; 87(11):1012-1019.[10] Brauchle E, Knopf A, Bauer H, et al. Non-invasive Chamber-Specific Identification of Cardiomyocytes in Differentiating Pluripotent Stem Cells. Stem Cell Reports. 2016;6(2):188-199.[11] Chan JW, Lieu DK, Huser T, et al. Label-free separation of human embryonic stem cells and their cardiac derivatives using Raman spectroscopy. Anal Chem. 2009;81(4): 1324-1331.[12] Ichimura T, Chiu LD, Fujita K, et al. Visualizing cell state transition using Raman spectroscopy. PLoS One. 2014; 9(1):e84478.[13] Pijanka JK, Kumar D, Dale T, et al. Vibrational spectroscopy differentiates between multipotent and pluripotent stem cells. Analyst. 2010;135(12):3126-3132.[14] Tsikritsis D, Shi H, Wang Y, et al. Label-free biomarkers of human embryonic stem cell differentiation to hepatocytes. Cytometry A. 2016;89(6):575-584.[15] 李芳,吕军强,段永娟,等.胎儿骨髓间充质干细胞对成人外周血淋巴细胞体外增殖及免疫相关因子表达的影响[J].中华血液学杂志, 2014, 35(10):891-896.[16] El-Kehdy H, Pourcher G, Zhang W, et al. Hepatocytic Differentiation Potential of Human Fetal Liver Mesenchymal Stem Cells: In Vitro and In Vivo Evaluation. Stem Cells Int. 2016;2016:6323486.[17] 袁源,王媛丽,杨树源,等.人脐带间质干细胞分离纯化及基本生物学特性研究[J].青岛大学医学院学报,2006,42(2):120-125.[18] Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145-1147.[19] 潘光锦,裴端卿.维持胚胎干细胞多能性的分子机制[J].生命科学,2007,19(4):372-377.[20] Ghita A, Pascut FC, Sottile V, et al. Applications of Raman micro-spectroscopy to stem cell technology: label-free molecular discrimination and monitoring cell differentiation. EPJ Tech Instrum. 2015;2(1):6.[21] Notingher I, Verrier S, Haque S, et al. Spectroscopic study of human lung epithelial cells (A549) in culture: living cells versus dead cells. Biopolymers. 2003;72(4):230-240.[22] Ilin Y, Choi JS, Harley BA, et al. Identifying States along the Hematopoietic Stem Cell Differentiation Hierarchy with Single Cell Specificity via Raman Spectroscopy. Anal Chem. 2015; 87(22):11317-11324.[23] Bai H, Li H, Han Z, et al. Label-free assessment of replicative senescence in mesenchymal stem cells by Raman microspectroscopy. Biomed Opt Express. 2015;6(11): 4493- 4500. [24] Butler HJ, Ashton L, Bird B, et al. Using Raman spectroscopy to characterize biological materials. Nat Protoc. 2016;11(4): 664-687.[25] Brivanlou AH, Gage FH, Jaenisch R, et al. Stem cells. Setting standards for human embryonic stem cells. Science. 2003; 300(5621):913-916.[26] Adewumi O, Aflatoonian B, Ahrlund-Richter L, et al. Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat Biotechnol. 2007;25(7): 803-816.[27] Klimanskaya I, Chung Y, Becker S, et al. Human embryonic stem cell lines derived from single blastomeres. Nature. 2006; 444(7118):481-485.[28] Kfoury Y, Scadden DT. Mesenchymal cell contributions to the stem cell niche. Cell Stem Cell. 2015;16(3):239-253.[29] Ankrum JA, Ong JF, Karp JM.Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014; 32(3):252-260.[30] James S, Fox J, Afsari F, et al. Multiparameter Analysis of Human Bone Marrow Stromal Cells Identifies Distinct Immunomodulatory and Differentiation-Competent Subtypes. Stem Cell Reports. 2015;4(6):1004-1015.[31] Lin D, Feng S, Pan J, et al. Colorectal cancer detection by gold nanoparticle based surface-enhanced Raman spectroscopy of blood serum and statistical analysis.Opt Express. 2011;19(14):13565-13577. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [3] | Xiao Hao, Liu Jing, Zhou Jun. Research progress of pulsed electromagnetic field in the treatment of postmenopausal osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1266-1271. |
| [4] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [5] | Wen Dandan, Li Qiang, Shen Caiqi, Ji Zhe, Jin Peisheng. Nocardia rubra cell wall skeleton for extemal use improves the viability of adipogenic mesenchymal stem cells and promotes diabetes wound repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1038-1044. |
| [6] | Zhu Bingbing, Deng Jianghua, Chen Jingjing, Mu Xiaoling. Interleukin-8 receptor enhances the migration and adhesion of umbilical cord mesenchymal stem cells to injured endothelium [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1045-1050. |
| [7] | Fang Xiaolei, Leng Jun, Zhang Chen, Liu Huimin, Guo Wen. Systematic evaluation of different therapeutic effects of mesenchymal stem cell transplantation in the treatment of ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1085-1092. |
| [8] | Guo Jia, Ding Qionghua, Liu Ze, Lü Siyi, Zhou Quancheng, Gao Yuhua, Bai Chunyu. Biological characteristics and immunoregulation of exosomes derived from mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1093-1101. |
| [9] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [10] | Tian Chuan, Zhu Xiangqing, Yang Zailing, Yan Donghai, Li Ye, Wang Yanying, Yang Yukun, He Jie, Lü Guanke, Cai Xuemin, Shu Liping, He Zhixu, Pan Xinghua. Bone marrow mesenchymal stem cells regulate ovarian aging in macaques [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 985-991. |
| [11] | Hou Jingying, Guo Tianzhu, Yu Menglei, Long Huibao, Wu Hao. Hypoxia preconditioning targets and downregulates miR-195 and promotes bone marrow mesenchymal stem cell survival and pro-angiogenic potential by activating MALAT1 [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1005-1011. |
| [12] | Liang Xuezhen, Yang Xi, Li Jiacheng, Luo Di, Xu Bo, Li Gang. Bushen Huoxue capsule regulates osteogenic and adipogenic differentiation of rat bone marrow mesenchymal stem cells via Hedgehog signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1020-1026. |
| [13] | Liu Feng, Peng Yuhuan, Luo Liangping, Wu Benqing. Plant-derived basic fibroblast growth factor maintains the growth and differentiation of human embryonic stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1032-1037. |
| [14] | Huang Chuanjun, Zou Yu, Zhou Xiaoting, Zhu Yangqing, Qian Wei, Zhang Wei, Liu Xing. Transplantation of umbilical cord mesenchymal stem cells encapsulated in RADA16-BDNF hydrogel promotes neurological recovery in an intracerebral hemorrhage rat model [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 510-515. |
| [15] | He Yunying, Li Lingjie, Zhang Shuqi, Li Yuzhou, Yang Sheng, Ji Ping. Method of constructing cell spheroids based on agarose and polyacrylic molds [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 553-559. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||