Chinese Journal of Tissue Engineering Research ›› 2017, Vol. 21 ›› Issue (13): 2036-2042.doi: 10.3969/j.issn.2095-4344.2017.13.012
Previous Articles Next Articles
Histological changes of bone marrow mesenchymal stem cells combined with Bio-oss in repairing rabbit skull defects
Shao Yan-lin1, 2, Luo Shi-jun3, Sun Song2, Sun Yong1, 2, Zhong Ke1, 2, Chen Hong-liang2
- 1School of Stomatology, Southwest Medical University, Luzhou 646000, Sichuan Province, China; 2Department of Stomatology, the Authority Hospital of Chengdu Military Region of PLA, Chengdu 610031, Sichuan Province, China; 3Department of Oral and Maxillofacial Surgery, Hospital of Stomatology, Southwest Medical University, Luzhou 646000, Sichuan Province, China
-
Revised:2017-03-21Online:2017-05-08Published:2017-06-09 -
Contact:Sun Yong, Professor, Chief physician, Master’s supervisor, School of Stomatology, Southwest Medical University, Luzhou 646000, Sichuan Province, China; Department of Stomatology, the Authority Hospital of Chengdu Military Region of PLA, Chengdu 610031, Sichuan Province, China -
About author:Shao Yan-lin, Studying for master’s degree, Physician, School of Stomatology, Southwest Medical University, Luzhou 646000, Sichuan Province, China; Department of Stomatology, the Authority Hospital of Chengdu Military Region of PLA, Chengdu 610031, Sichuan Province, China -
Supported by:the “Twelfth Five-Year” Projects of Chengdu Military Region, No. C14050
CLC Number:
Cite this article
Shao Yan-lin, Luo Shi-jun, Sun Song, Sun Yong, Zhong Ke, Chen Hong-liang. Histological changes of bone marrow mesenchymal stem cells combined with Bio-oss in repairing rabbit skull defects[J]. Chinese Journal of Tissue Engineering Research, 2017, 21(13): 2036-2042.
share this article
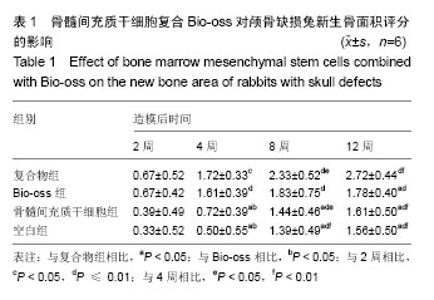
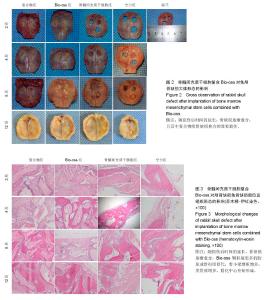
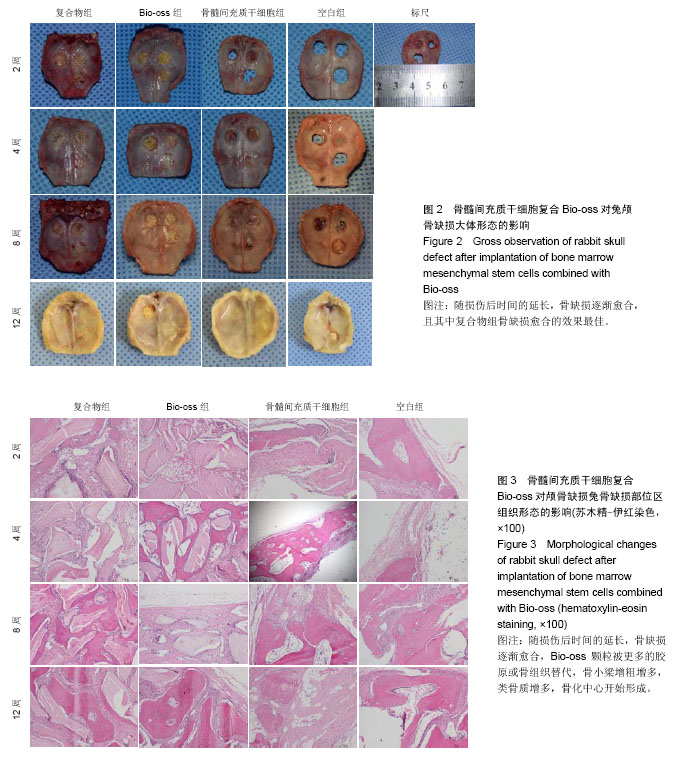
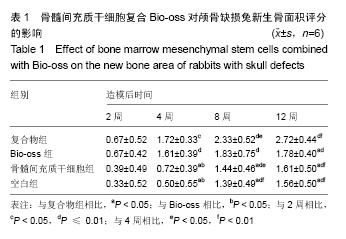
2.1 实验动物数量分析 纳入的96只日本大耳兔均进入结果分析,无脱失。 2.2 大体观察结果 造模后2周,复合物组和Bio-oss组可见Bio-oss颗粒明显,质地中等,表面呈粗糙颗粒状,与自体骨界限清晰。骨髓间充质干细胞组和空白组骨缺损边缘见少量新生组织,表面光滑的,质地软,边界较清晰(图2)。 造模后4周,复合物组和Bio-oss组可见Bio-oss颗粒,与2周时相比新生组织增多,质地中等,与周围边界清晰,表面颗粒状,材料表面可见纤维组织贴附生长。骨髓间充质干细胞组和空白组新生组织面积比2周大,表面光滑,向中央呈圈状融合生长,质地较软,周界较清晰(图2)。 造模后8周,复合物组和Bio-oss组Bio-oss颗粒部分降解,与自体骨边界不清晰,表面较光滑,质地较硬,骨髓间充质干细胞组边缘新生组织向中央呈圈状融合生长,面积与4周时相比增大,中央有少量缺损,新生组织表面光滑稍凹陷,质稍硬,边界不清,空白组缺损由新生组织填充,无明显缺损,质地稍硬,与自体骨边界不清,表面光滑稍凹陷(图2)。 造模后12周,复合物组和Bio-oss组Bio-oss颗粒进一步降解,与8周时相比颗粒更少,质地硬,边界不清,表面较光滑,有结缔组织长入。骨髓间充质干细胞组和空白组无组织缺损,新生组织质地中等,骨髓间充质干细胞组和空白组相比,骨髓间充质干细胞组表面光滑稍凹陷,质稍硬,边界不清(图2)。 引导组织再生膜均无明显移位,2周时引导组织再生膜完整,4周时明显比2周薄,8周时降解明显,仅存留小部分,12周时膜基本完全降解。 2.3 组织学观察结果 造模后2周,复合物组和Bio-oss组骨缺损部位区可见大量Bio-oss颗粒,周围炎性细胞浸润,胶原纤维增生,排列紊乱;骨髓间充质干细胞组和空白组炎性细胞浸润,毛细血管丰富,胶原纤维大量增生(图3)。造模后4周,复合物组和Bio-oss组术区可见部分Bio-oss颗粒被纤维结缔组织分割,Bio-oss边缘见多核细胞和成骨细胞黏附,类骨质生成,少量新生血管,血管直径约为25 μm[22],没有成熟骨小梁和矿化骨生成;骨髓间充质干细胞组和空白组见少量新生血管、类骨质和胶原纤维(图3)。造模后8周,复合物组和Bio-oss组Bio-oss颗粒进一步降解,可见增生活跃的成骨细胞,新生骨逐渐成熟、矿化但排列紊乱,新生血管较多,能见骨化中心,有很细小的骨小梁生成;骨髓间充质干细胞组和空白组出现少量成熟骨,多数仍为不成熟骨,有细小骨小梁生成(图3)。造模后12周,复合物组和Bio-oss组Bio-oss颗粒被更多的胶原或骨组织替代,骨小梁生成更多且逐渐加粗,骨质更成熟,较多新生血管,骨化中心增多;骨髓间充质干细胞组和空白组骨小梁增粗增多,类骨质增多,骨化中心开始形成(图3)。造模后2周,4组新生骨面积接近;造模后4周,复合物组和Bio-oss组新生骨面积显著优于骨髓间充质干细胞组和空白组(P < 0.05);造模后8周,复合物组新生骨面积显著优于骨髓间充质干细胞组和空白组(P < 0.05);造模后12周,复合物组新生骨面积显著优于其他3组(P < 0.05;表1)。复合物组中造模后4,8,12周时的新生骨面积均显著优于2周(P < 0.05),且8,12周时的新生骨面积显著多于4周(P < 0.05);Bio-oss组中造模后4,8,12周时的新生骨面积显著优于2周(P < 0.01);骨髓间充质干细胞组和空白组中造模后8和12周时的新生骨面积显著优于2和4周(P < 0.01;表1)。"
| [1] Lambrecht JT.口腔外科与种植外科[M].胡开进,译.北京:人民军医出版社,2014.[2] Moroni L, Schotel R, Sohier J, et al. Polymer hollow fiber three-dimensional matrices with controllable cavity and shell thickness. Biomaterials. 2006;27(35):5918-5926.[3] Liu X, Liao X, Luo E, et al. Mesenchymal stem cells systemically injected into femoral marrow of dogs home to mandibular defects to enhance new bone formation. Tissue Eng Part A. 2014;20(3-4):883-892.[4] Henkel J, Woodruff MA, Epari DR, et al. Bone Regeneration Based on Tissue Engineering Conceptions - A 21st Century Perspective. Bone Res. 2013;1(3):216-248.[5] 张俊标,何志旭,叶川,等.3-羟基丁酸-4-羟基丁酸共聚酯负载人骨髓间充质干细胞在骨组织工程中的应用[J].中国组织工程研究,2016,20(21):3057-3064.[6] Jayasuriya AC, Shah C, Ebraheim NA, et al. Acceleration of biomimetic mineralization to apply in bone regeneration. Biomed Mater. 2008;3(1):015003. [7] 黄江鸿,杨雷,廖福朋,等.低氧诱导因子-1α诱导分化的骨髓间充质干细胞复合纳米人工骨修复兔桡骨缺损[J].骨科,2016,7(3): 201-206,212.[8] Löfgren H, Engquist M, Hoffmann P, et al. Clinical and radiological evaluation of Trabecular Metal and the Smith-Robinson technique in anterior cervical fusion for degenerative disease: a prospective, randomized, controlled study with 2-year follow-up. Eur Spine J. 2010;19(3):464-473.[9] Jaiswal N, Haynesworth SE, Caplan AI, et al. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64(2): 295-312.[10] Vitale-Brovarone C, Ciapetti G, Leonardi E, et al. Resorbable glass-ceramic phosphate-based scaffolds for bone tissue engineering: synthesis, properties, and in vitro effects on human marrow stromal cells. J Biomater Appl. 2011;26(4): 465-489.[11] 张彬,湛梅圣,赵玉玺,等.BMSCs-BMP2-煅烧牛骨复合物的制作及其在兔尺骨骨缺损修复中的应用[J].山东医药,2015,55(12): 17-20,111.[12] Liu X, Liao X, Luo E, et al. Mesenchymal stem cells systemically injected into femoral marrow of dogs home to mandibular defects to enhance new bone formation. Tissue Eng Part A. 2014;20(3-4):883-892.[13] Zhou Q, Yu BH, Liu WC, et al. BM-MSCs and Bio-Oss complexes enhanced new bone formation during maxillary sinus floor augmentation by promoting differentiation of BM-MSCs. In Vitro Cell Dev Biol Anim. 2016;52(7):757-771.[14] Yang C, Liu Y, Li C, et al. Repair of mandibular defects by bone marrow stromal cells expressing the basic fibroblast growth factor transgene combined with multi-pore mineralized Bio-Oss. Mol Med Rep. 2013;7(1):99-104.[15] 赵健,黄远亮,刘阿贵,等.以Bio-oss胶原为支架材料的组织工程化骨修复种植体周围骨缺损的初步实验研究[J].口腔医学,2013, 33(2):95-99.[16] 谭丽思,林晓萍,刘尧,等.组织工程化骨修复犬牙槽骨缺损的组织学观察[J].解剖科学进展,2010,16(6):492-495.[17] 谭丽思,林晓萍,刘尧,等.组织工程骨促进犬牙槽骨再生的体内研究[J].口腔医学研究,2010,26(2):183-186.[18] 李京旭,李龙和,玄云泽.Bio-oss/PRF复合支架结合骨髓基质细胞构建组织工程骨实验研究[J].延边大学医学学报,2014, 37(4): 235-238.[19] 赵健,黄远亮,蒋欣泉,等.Bio-Oss胶原结合骨髓基质细胞构建组织工程骨的实验研究[J].中国口腔颌面外科杂志,2006,4(3):215-219.[20] 谭志军,陈艳,张翠萍,等.微小RNA调控骨髓间充质干细胞分化的研究进展[J].中华实验外科杂志,2014,31(6):1389-1390.[21] 罗世君,吴倩倩,孙勇,等.兔骨髓间充质干细胞的分离培养及成骨分化的实验研究[J].西南国防医药,2016,26(6):590-593.[22] Song G, Habibovic P, Bao C, et al. The homing of bone marrow MSCs to non-osseous sites for ectopic bone formation induced by osteoinductive calcium phosphate. Biomaterials. 2013;34(9):2167-2176.[23] Lyons FG, Gleeson JP, Partap S, et al. Novel microhydroxyapatite particles in a collagen scaffold: a bioactive bone void filler? Clin Orthop Relat Res. 2014;472(4):1318-1328.[24] 吴凯.BMP-2和BMSCs结合n-HA/n-LDIG复合材料修复兔桡骨缺损的实验研究[D].长沙:中南大学,2013.[25] Zhu H, Jiang XX, Wu Y, et al. Identification of mesenchymal stem cells derived from rheumatoid arthritis synovial fluid and their regulatory effect on osteoblast formation. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2009;17(4):977-980.[26] Reinshagen H, Auw-Haedrich C, Sorg RV, et al. Corneal surface reconstruction using adult mesenchymal stem cells in experimental limbal stem cell deficiency in rabbits. Acta Ophthalmol. 2011;89(8):741-748.[27] Huang L, Li R, Liu W, et al. Dynamic culture of a thermosensitive collagen hydrogel as an extracellular matrix improves the construction of tissue-engineered peripheral nerve. Neural Regen Res. 2014;9(14):1371-1378.[28] 韩操,马宁,李忠义,等.自体骨髓间充质干细胞载体复合物修复骨缺损的组织学观察[J].中国组织工程研究,2015,19(6):891-897.[29] 董红宾,张琴,何惠宇,等.三种异种骨材料修复牙周骨缺损的比较[J].中国组织工程研究,2015,19(8):1170-1176.[30] Duda M, Pajak J. The issue of bioresorption of the Bio-Oss xenogeneic bone substitute in bone defects. Ann Univ Mariae Curie Sklodowska Med. 2004;59(1):269-277.[31] Du C, Yao C, Li N, et al. Cell sheet-engineered bones used for the reconstruction of mandibular defects in an animal model. Exp Ther Med. 2015;10(6):2216-2220.[32] Kim HC, Song JM, Kim CJ, et al. Combined effect of bisphosphonate and recombinant human bone morphogenetic protein 2 on bone healing of rat calvarial defects. Maxillofac Plast Reconstr Surg. 2015;37(1):16.[33] Arvidson K, Abdallah BM, Applegate LA, et al. Bone regeneration and stem cells. J Cell Mol Med. 2011;15(4): 718-746.[34] Chatterjea A, van der Stok J, Danoux CB, et al. Inflammatory response and bone healing capacity of two porous calcium phosphate ceramics in critical size cortical bone defects. J Biomed Mater Res A. 2014;102(5):1399-1407.[35] Kang SH, Chung YG, Oh IH, et al. Bone regeneration potential of allogeneic or autogeneic mesenchymal stem cells loaded onto cancellous bone granules in a rabbit radial defect model. Cell Tissue Res. 2014;355(1):81-88.[36] Liu T, Wu G, Wismeijer D, et al. Deproteinized bovine bone functionalized with the slow delivery of BMP-2 for the repair of critical-sized bone defects in sheep. Bone. 2013;56(1): 110-118.[37] Wehrhan F, Amann K, Molenberg A, et al. Critical size defect regeneration using PEG-mediated BMP-2 gene delivery and the use of cell occlusive barrier membranes - the osteopromotive principle revisited. Clin Oral Implants Res. 2013;24(8):910-920.[38] Choi S, Liu IL, Yamamoto K, et al. Implantation of tetrapod-shaped granular artificial bones or β-tricalcium phosphate granules in a canine large bone-defect model. J Vet Med Sci. 2014;76(2):229-235.[39] Seeherman HJ, Li XJ, Smith E, et al. rhBMP-2/calcium phosphate matrix induces bone formation while limiting transient bone resorption in a nonhuman primate core defect model. J Bone Joint Surg Am. 2012;94(19):1765-1776.[40] Schlegel KA, Lang FJ, Donath K, et al. The monocortical critical size bone defect as an alternative experimental model in testing bone substitute materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(1):7-13.[41] Schmitz JP, Hollinger JO. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin Orthop Relat Res. 1986;(205):299-308.[42] Gosain AK, Song L, Yu P, et al. Osteogenesis in cranial defects: reassessment of the concept of critical size and the expression of TGF-beta isoforms. Plast Reconstr Surg. 2000; 106(2):360-371; discussion 372.[43] Jiang ZQ, Liu HY, Zhang LP, et al. Repair of calvarial defects in rabbits with platelet-rich plasma as the scaffold for carrying bone marrow stromal cells. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113(3):327-333.[44] 傅涛.不同粒径GP/CPC人工骨修复新西兰兔颅骨缺损效果的对比研究[D].杭州:浙江大学,2014.[45] 陈畅行,宋晓陵,达静姝,等.两种脱细胞真皮基质材料在兔颅骨缺损引导骨再生术中的成骨效果评估[J].牙体牙髓牙周病学杂志, 2014,24(5):274-277.[46] 李耘.明胶微粒粒径与配比对GP/CPC复合人工骨材料修复骨缺损的影响[D].杭州:浙江大学,2015.[47] 王媛,王稚英,王明.同种异体牙源性骨移植材料修复兔颅骨缺损的实验研究[J].中国医科大学学报,2016,45(12):1082-1085.[48] Naito Y, Terukina T, Galli S, et al. The effect of simvastatin-loaded polymeric microspheres in a critical size bone defect in the rabbit calvaria. Int J Pharm. 2014;461(1-2): 157-162.[49] Li G, Wang X, Cao J, et al. Coculture of peripheral blood CD34+ cell and mesenchymal stem cell sheets increase the formation of bone in calvarial critical-size defects in rabbits. Br J Oral Maxillofac Surg. 2014;52(2):134-139. |
| [1] | Yao Xiaoling, Peng Jiancheng, Xu Yuerong, Yang Zhidong, Zhang Shuncong. Variable-angle zero-notch anterior interbody fusion system in the treatment of cervical spondylotic myelopathy: 30-month follow-up [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(9): 1377-1382. |
| [2] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [3] | Xiao Hao, Liu Jing, Zhou Jun. Research progress of pulsed electromagnetic field in the treatment of postmenopausal osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1266-1271. |
| [4] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [5] | Wen Dandan, Li Qiang, Shen Caiqi, Ji Zhe, Jin Peisheng. Nocardia rubra cell wall skeleton for extemal use improves the viability of adipogenic mesenchymal stem cells and promotes diabetes wound repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1038-1044. |
| [6] | Zhu Bingbing, Deng Jianghua, Chen Jingjing, Mu Xiaoling. Interleukin-8 receptor enhances the migration and adhesion of umbilical cord mesenchymal stem cells to injured endothelium [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1045-1050. |
| [7] | Cui Xing, Sun Xiaoqi, Zheng Wei, Ma Dexin. Huangqin Decoction regulates autophagy to intervene with intestinal acute graft-versus-host disease in mice [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1057-1062. |
| [8] | Fang Xiaolei, Leng Jun, Zhang Chen, Liu Huimin, Guo Wen. Systematic evaluation of different therapeutic effects of mesenchymal stem cell transplantation in the treatment of ischemic stroke [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1085-1092. |
| [9] | Guo Jia, Ding Qionghua, Liu Ze, Lü Siyi, Zhou Quancheng, Gao Yuhua, Bai Chunyu. Biological characteristics and immunoregulation of exosomes derived from mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1093-1101. |
| [10] | Zhang Jinglin, Leng Min, Zhu Boheng, Wang Hong. Mechanism and application of stem cell-derived exosomes in promoting diabetic wound healing [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1113-1118. |
| [11] | Tian Chuan, Zhu Xiangqing, Yang Zailing, Yan Donghai, Li Ye, Wang Yanying, Yang Yukun, He Jie, Lü Guanke, Cai Xuemin, Shu Liping, He Zhixu, Pan Xinghua. Bone marrow mesenchymal stem cells regulate ovarian aging in macaques [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 985-991. |
| [12] | Hu Wei, Xie Xingqi, Tu Guanjun. Exosomes derived from bone marrow mesenchymal stem cells improve the integrity of the blood-spinal cord barrier after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 992-998. |
| [13] | Hou Jingying, Guo Tianzhu, Yu Menglei, Long Huibao, Wu Hao. Hypoxia preconditioning targets and downregulates miR-195 and promotes bone marrow mesenchymal stem cell survival and pro-angiogenic potential by activating MALAT1 [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1005-1011. |
| [14] | Liang Xuezhen, Yang Xi, Li Jiacheng, Luo Di, Xu Bo, Li Gang. Bushen Huoxue capsule regulates osteogenic and adipogenic differentiation of rat bone marrow mesenchymal stem cells via Hedgehog signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1020-1026. |
| [15] | Huang Chuanjun, Zou Yu, Zhou Xiaoting, Zhu Yangqing, Qian Wei, Zhang Wei, Liu Xing. Transplantation of umbilical cord mesenchymal stem cells encapsulated in RADA16-BDNF hydrogel promotes neurological recovery in an intracerebral hemorrhage rat model [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(4): 510-515. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||