Chinese Journal of Tissue Engineering Research ›› 2016, Vol. 20 ›› Issue (41): 6216-6224.doi: 10.3969/j.issn.2095-4344.2016.41.021
Previous Articles Next Articles
Research progress in immunomodulatory properties of mesenchymal stem cells
Yi Qiao1, 2, Lu Yan-qin1, 2, Huang Hong-yu1, 2, Zhou Dian1, 2, Liu Ou-sheng1,2
- 1Xiangya Stomatology Hospital, Central South University, Changsha 410078, Hunan Province, China
2Central South University, Changsha 410078, Hunan Province, China
-
Revised:2016-08-13Online:2016-10-07Published:2016-10-07 -
Contact:Liu Ou-sheng, M.D., Master’s supervisor, Xiangya Stomatology Hospital, Central South University, Changsha 410078, Hunan Province, China; Central South University, Changsha 410078, Hunan Province, China -
About author:Yi Qiao, Master, Xiangya Stomatology Hospital, Central South University, Changsha 410078, Hunan Province, China; Central South University, Changsha 410078, Hunan Province, China -
Supported by:the Fundamental Research Funds for the Central Universities in Central South University, No. 2014zztx306; the National Natural Science Foundation of China, No. 81300841
CLC Number:
Cite this article
Yi Qiao, Lu Yan-qin, Huang Hong-yu, Zhou Dian, Liu Ou-sheng. Research progress in immunomodulatory properties of mesenchymal stem cells[J]. Chinese Journal of Tissue Engineering Research, 2016, 20(41): 6216-6224.
share this article
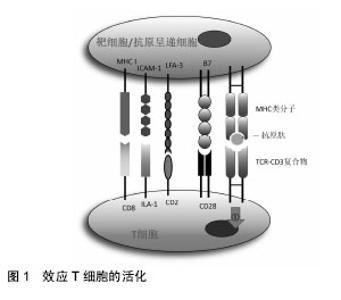
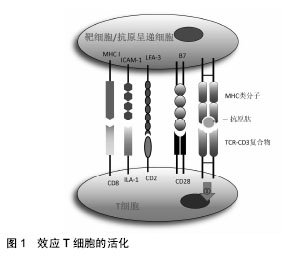
2.1 间充质干细胞对T细胞的免疫调节作用 T细胞是来源于骨髓的多能干细胞,在胸腺内分化成熟,是淋巴细胞中数量最多、功能最复杂的一类细胞。在无抗原激发的情况下,效应T细胞是以不活化的静息型细胞形式存在。当抗原进入机体后,在抗原呈递细胞或靶细胞的作用下使静息型T细胞活化增殖并分化为效应T细胞发挥细胞免疫作用。效应性T细胞的活化需要双信号,即效应性T细胞与抗原递呈细胞或靶细胞膜上MHC类分子和抗原肽分子复合物结合后,可通过CD3复合分子传递第一信号;而效应性体T细胞上的其他辅助分子如CD2,LFA-1、CD8及CD28分子等可与抗原递呈细胞或靶细胞上相应的配体分子如LFA-3、ICAM-1、MHCⅠ类分子及B7分子等结合,向效应性T细胞传递协同信号并使之活化(图1)。"
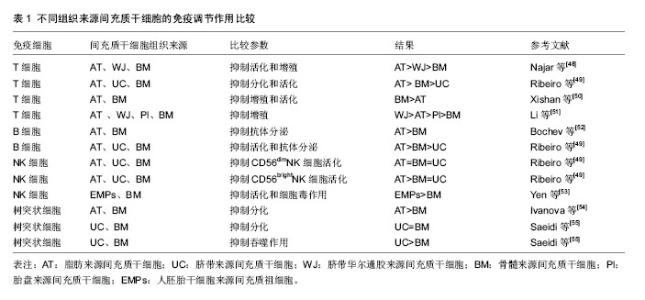
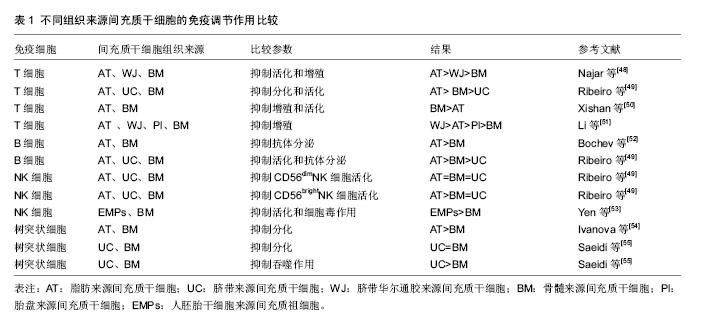
间充质干细胞抑制T细胞分裂增殖,近年来被反复证明间充质干细胞可阻断T细胞周期,使T细胞停留在G0/G1期[10-13]。在分子层面上,细胞周期素表达升高,而P27表达降低[10-11]。而对于间充质干细胞是否诱导T细胞凋亡,不同的研究得出了不同的结论。有研究认为间充质干细胞对T细胞的凋亡没有影响[14],但也有研究认为间充质干细胞可以诱导T细胞的凋亡[15-17],甚至有研究认为间充质干细胞对T细胞凋亡有保护作用[18]。同时,有研究表明间充质干细胞还可以抑制T细胞的活化作用[19]。总之,有关间充质干细胞对T细胞免疫抑制的机制还需要进一步的研究证明。 间充质干细胞对T细胞的作用主要通过可溶性细胞因子实现。Transwell实验的应用,证明了可溶性细胞因子在间充质干细胞抑制T细胞增殖过程中的重要作用[20-21]。Soleymaninejadian等[22]对与间充质干细胞免疫调节作用相关的细胞因子做一综述,其中主要包括转化生长因子β、肝细胞生长因子、前列腺素E2、白细胞介素10、吲哚胺2,3-双加氧酶(indolamine 2,3-dioxygenase,IDO)、一氧化氮、血红素氧合酶1、HLA-G等。吲哚胺2,3-双加氧酶参与色氨酸代谢,并导致色氨酸缺乏,从而抑制T细胞活性。一氧化氮则通过抑制树突状细胞成熟和肿瘤坏死因子α分泌从而抑制T细胞活性。HLA-G通过与受体的相互作用抑制T细胞活性。 间充质干细胞与T细胞的相互作用不仅与可溶性因子的作用有关,也与细胞间相互作用有关。Nicola等[20]首先发现在间充质干细胞与T细胞接触的条件下比在Transwell条件下对T细胞的免疫抑制作用强,提示了细胞接触在间充质干细胞与T细胞相互作用中的重要作用。在间充质干细胞与T细胞相互接触过程中,间充质干细胞主要通过程序性死亡因子1与其配体相互作用抑制T细胞增殖[23]。此外,Sheng等[24]研究发现,间充质干细胞表达B7-H1分子,与CD28相互作用后,阻断了T细胞的协同刺激通路,抑制了免疫反应。其他研究也得出了相似的结论,即间充质干细胞通过细胞接触抑制T细胞的增殖[25-26]。 间充质干细胞对T细胞的抑制作用还具有浓度依赖性[20,27],间充质干细胞︰T细胞的比率越高,对T细胞的抑制作用越强。还有研究显示间充质干细胞︰T细胞的比率降到一定程度时还能表现一定的免疫刺激作用。这种免疫刺激作用主要发生在CD3/CD28抗体刺激的T细胞与间充质干细胞共培养的实验中。研究者认为CCL2在间充质干细胞︰T细胞低比率(10︰1)的情况下对T细胞的刺激起到了重要作用。CCL2通过表达于活化T细胞的CCR2相互作用刺激T细胞增殖[28]。 2.2 间充质干细胞对B细胞的免疫调节作用 B细胞是来源于骨髓的多能干细胞,在抗原刺激和T细胞辅助下,大量增殖并进一步分化发育成为能够分泌抗体的终末细胞——浆细胞。 间充质干细胞对B细胞的增殖和分化具有一定的抑制作用[29-30]。间充质干细胞在IFN-γ的作用下抑制B细胞增殖和分化,且IFN-γ在此相互作用中有关键作用。Ren等[31]研究发现,从缺乏IFN-γ或IFN-γ受体的小鼠中产生的间充质干细胞对B细胞没有抑制作用。但也有研究得出不同结论,间充质干细胞能够通过细胞接触作用诱导B细胞增殖和分化[32-33]。这些存在矛盾的实验结果可能与细胞提纯方式、培养环境和检测时间点等有关,尚需进一步研究。 除此之外,B细胞分泌抗体和表达趋化因子受体CXCR4、CXCR5、CCR7的功能也受到了间充质干细胞的抑制,而B细胞共刺激分子和细胞因子的分泌并不受间充质干细胞的影响[34]。 间充质干细胞对B细胞的作用是通过细胞接触实现的,且PD-1/PDL-1途径是间充质干细胞对B细胞抑制的重要途径[29]。曾有研究显示PD-1可通过聚集磷酸酶去磷酸化一些BCR信号传导的关键信号传导器,从而阻碍BCR信号传导,抑制B细胞活化[35]。此外,有研究提示T细胞在间充质干细胞对B细胞的免疫调节中起了重要作用,间充质干细胞抑制B细胞增殖和分化需要T细胞与间充质干细胞间细胞接触的相互作用才能实现[30]。 间充质干细胞调节B细胞功能除了细胞接触的关键作用外,可溶性细胞因子也起到了不可忽视的作用。研究发现,间充质干细胞分泌产生CCL2,并通过抑制转录激活蛋白3并诱导产生双链复合蛋白5(paired box protein,PAX5)实现对B细胞的免疫调节功能[36]。Che等[37]研究发现,在间充质干细胞共培养体系中,Blimp-1的mRNA表达明显降低,PAX-5的mRNA表达增加,由此认为通过抑制Blimp-1表达和促进PAX-5表达对B细胞增殖和分化产生抑制作用。 2.3 间充质干细胞对NK细胞的免疫调节作用 NK细胞是自然杀伤细胞的简称,它不同于T细胞,也不同于B细胞,对靶细胞杀伤时既不需特异性抗体参加,也不需抗原特异性致敏,是机体固有免疫的主要承担者。NK细胞通过识别靶细胞上相应活化受体和抑制性受体的配体而区分正常和异常细胞,发挥其免疫监视作用。 有很多研究报道,间充质干细胞能够抑制NK细胞的增殖和功能[38-40]。NK细胞受具有激活作用的细胞因子刺激,导致NK细胞表面激活受体NKp30、NKG2D、NKp44、CD69高表达,这些细胞表面激活受体与NK细胞的功能密切相关。Spaggiari等[38]研究发现,NK细胞与间充质干细胞共培养,NK细胞激活受体NKp30与NKG2D表达降低,不表达NKp44,由此推断间充质干细胞通过抑制NK细胞激活受体的表达从而抑制NK细胞的细胞毒作用。此外,Spaggiari等[41]通过mAb掩饰实验阻断NKp30,NKG2D 和 DNAM-1的表达,发现NK细胞的细胞毒作用受到了抑制。 IFN-γ在间充质干细胞对NK细胞的抑制作用中起到了关键作用。间充质干细胞对NK细胞毒作用的敏感性可因IFN-γ的存在而降低[42]。IFN-γ的作用可能是诱导间充质干细胞高表达HLA-Ⅰ类分子,并与NK细胞的抑制受体相互作用,从而抑制NK细胞的细胞毒作用[41-42]。在Transwell实验中,间充质干细胞仍能对NK细胞产生抑制作用,表明间充质干细胞通过可溶性因子对NK细胞产生抑制作用[39]。其中吲哚胺2,3-双加氧酶、前列腺素E2、HLA-G在间充质干细胞对NK细胞的抑制作用中发挥了主要作用。HLA-G 受体表达在白细胞表面,称为LILRB,可表达于T、B和NK细胞表面,HLA-G/LILRB2的相互作用阻断MEK/ERK信号通路抑制NK细胞的细胞毒作用[43]。在间充质干细胞与NK细胞混合培养中加入吲哚胺2,3-双加氧酶或前列腺素E2在一定程度上均能恢复NK细胞的增殖,且二者有协同作用[38]。在IFN-γ和肿瘤坏死因子α的存在下,间充质干细胞与NK细胞相互作用,间充质干细胞分泌前列腺素E2,随后,前列腺素E2能够促进吲哚胺2,3-双加氧酶的合成。此外,间充质干细胞对NK细胞的抑制作用具有浓度依赖性。随着NK/间充质干细胞的比率越高,间充质干细胞对NK细胞的抑制作用越弱。 2.4 间充质干细胞对树突状细胞的免疫调节作用 树突状细胞是重要的抗原提呈细胞,来源于多能造血干细胞。非成熟树突状细胞具有极强的抗原摄取、加工和处理能力。在摄取抗原或受到某些刺激后,非成熟树突状细胞(CD14-,CD1a+)可以分化成熟树突状细胞(CD80+,CD83+,CD86+),在此过程中其抗原摄取和加工能力显著降低,抗原提呈和激发免疫应答的能力增强。树突状细胞通过其膜表面丰富的抗原肽-MHC Ⅰ类分子复合物、抗原肽-MHC Ⅱ类分子复合物提呈给相应的CD8+T细胞和CD4+T细胞,激活T细胞应答反应,并为T细胞提供共刺激分子,充分活化T细胞。 间充质干细胞能够抑制多能造血干细胞向树突状细胞分化和树突状细胞的成熟[44-46]。Jiang等[44]研究发现,在粒细胞-巨噬细胞刺激因子/白细胞介素4的刺激下,间充质干细胞与单核细胞共培养,CD1a表达降低,而CD14高表达,表明树突状细胞的分化受到抑制。此外,在脂多糖刺激下,间充质干细胞与树突状细胞共培养,树突状细胞的成熟标志CD83表达降低,树突状细胞表面共刺激分子CD80、CD86表达降低,抗原递呈分子HLA-DR表达降低,白细胞介素2分泌减少,表明间充质干细胞抑制树突状细胞成熟。间充质干细胞/单核细胞的比率较高时(1︰10),间充质干细胞对单核细胞向树突状细胞分化的抑制作用可完全通过可溶性因子实现。在间充质干细胞/单核细胞比率低时(1︰20或1︰40),间充质干细胞主要通过细胞接触发挥抑制作用。Nauta等[45]也得出了相似结论,间充质干细胞能够抑制CD34+来源CD14+前体细胞和单核细胞向CD1a+树突状细胞分化,并且抑制树突状细胞的成熟和功能。加入巨噬细胞集落刺激因子和白细胞介素6抗体,能降低CD14的表达,但不能恢复CD1a的表达,提示巨噬细胞集落刺激因子和白细胞介素6参与间充质干细胞的抑制作用。Spaggiari等[46]研究发现,加入前列腺素E2抑制剂NS-398能够恢复间充质干细胞对树突状细胞分化和功能的抑制。进一步研究发现,在单核细胞培养系中加入前列腺素E2能够起到与间充质干细胞一样的抑制其向树突状细胞分化的效果,不同于Nauta等发现的巨噬细胞集落刺激因子和白细胞介素6参与树突状细胞分化抑制的结论。 间充质干细胞对树突状细胞分化和成熟的抑制作用,间接导致T细胞活化受到抑制,从而抑制适应性免疫反应。间充质干细胞通过细胞接触和可溶性因子对树突状细胞产生抑制作用,可能还有巨噬细胞集落刺激因子和白细胞介素6和前列腺素E2的参与,具体机制尚待进一步研究。 2.5 不同组织来源间充质干细胞的免疫调节作用 间充质干细胞能够从骨髓、脂肪、脐血、皮肤、胎盘、牙髓等组织中分离而得,不同组织来源的间充质干细胞对其免疫调节作用是否存在影响呢?对于这一问题,不同研究结果之间存在一定的争议(表1)。 不同组织来源间充质干细胞对T细胞的免疫调节作用。Yoo等[47]研究发现,脂肪来源间充质干细胞、脐带来源间充质干细胞和脐带华尔通胶来源间充质干细胞与骨髓间充质干细胞具有相似免疫表型,非骨髓来源间充质干细胞对细胞分裂素激活的T细胞的免疫抑制作用均能达到骨髓间充质干细胞的水平,且部分免疫调节机制类似。有研究显示,脐带华尔通胶、脂肪和骨髓3种不同组织来源的间充质干细胞对T细胞增殖的抑制作用相比较,其中脂肪来源间充质干细胞对T细胞的抑制作用最强,而后依次为脐带华尔通胶来源间充质干细胞和骨髓间充质干细胞[48]。类似的,Ribeiro等[49]研究发现,脂肪来源间充质干细胞相较于脐带来源间充质干细胞和骨髓间充质干细胞,其对T细胞的免疫抑制作用最强。相反,Xishan等[50]研究发现,骨髓间充质干细胞对T细胞的免疫抑制作用强于脂肪来源间充质干细胞。此外,Li等[51]将脐带华尔通胶、胎盘、脂肪和骨髓4种不同组织来源间充质干细胞对T细胞增殖的抑制作用相比较,发现脐带华尔通胶来源间充质干细胞对T细胞的抑制作用最强,而骨髓间充质干细胞最弱[51]。 不同组织来源间充质干细胞对B细胞的免疫调节作用。Bochev等[52]研究发现,脂肪来源间充质干细胞对B细胞的功能和抗体分泌能力的抑制作用强于骨髓间充质干细胞。此外,Ribeiro等[49]对脂肪、骨髓和脐带3种组织来源间充质干细胞的B细胞免疫调节作用做一对比研究,同样发现脂肪来源间充质干细胞对B细胞抗体分泌能力的抑制作用强于骨髓间充质干细胞,而脐带来源间充质干细胞对B细胞的抗体分泌能力无影响。 不同组织来源间充质干细胞对NK细胞的免疫调节作用。Ribeiro等[49]同时还比较了脂肪、骨髓和脐带3种组织来源间充质干细胞对NK细胞的免疫调节作用。研究发现3种来源间充质干细胞对CD56dimNK细胞都具有免疫抑制作用,其中脂肪来源间充质干细胞的抑制作用最为显著,但脐带来源间充质干细胞对CD56brightNK细胞没有免疫抑制作用。此外,Yen等[53]比较了胚胎干细胞来源间叶祖细胞和骨髓间充质干细胞对NK细胞的免疫调节作用,发现胚胎干细胞来源间叶祖细胞对NK细胞的免疫抑制作用更强。 不同组织来源间充质干细胞对树突状细胞的免疫调节作用。Ivanova等[54]研究发现,脂肪来源间充质干细胞对树突状细胞分化能力的抑制作用比骨髓间充质干细胞更为显著。此外,Saeidi等[55]发现脐带来源间充质干细胞和骨髓间充质干细胞都能抑制树突状细胞的分化成熟且二者之间没有差别,但脐带来源间充质干细胞对树突状细胞吞噬作用的抑制作用更强。"
| [1] In 't Anker PS, Scherjon SA, Kleijburg-van der Keur C, et al. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood. 2003; 102(4):1548-1549. [2] Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109(1):235-242. [3] Gronthos S, Mankani M, Brahim J, et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97(25):13625- 13630. [4] Gronthos S, Franklin DM, Leddy HA, et al. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001; 189(1):54-63. [5] Friedenstein AJ, Petrakova KV, Kurolesova AI, et al. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6(2):230-247. [6] Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309): 71-74. [7] Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317. [8] Schüle S, Berger A. Mesenchymal stromal cells in the treatment of graft-versus-host disease: where do we stand. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2015;58(11-12):1265-1273. [9] Al Jumah MA, Abumaree MH. The immunomodulatory and neuroprotective effects of mesenchymal stem cells (MSCs) in experimental autoimmune encephalomyelitis (EAE): a model of multiple sclerosis (MS). Int J Mol Sci. 2012;13(7):9298-9331. [10] Glennie S, Soeiro I, Dyson PJ, et al. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105(7):2821-2827. [11] Sato K, Ozaki K, Oh I, et al. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109(1):228-234. [12] Hao L, Zhang C, Chen XH, et al. Human umbilical cord blood-derived stromal cells suppress xenogeneic immune cell response in vitro. Croat Med J. 2009;50(4): 351-360. [13] Hu J, Zhang X, Zhou L, et al. Immunomodulatory properties of colonic mesenchymal stem cells. Immunol Lett. 2013;156(1-2):23-29. [14] Zappia E, Casazza S, Pedemonte E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106(5):1755-1761. [15] Plumas J, Chaperot L, Richard MJ, et al. Mesenchymal stem cells induce apoptosis of activated T cells. Leukemia. 2005;19(9):1597-1604. [16] Fournel S, Aguerre-Girr M, Huc X, et al. Cutting edge: soluble HLA-G1 triggers CD95/CD95 ligand-mediated apoptosis in activated CD8+ cells by interacting with CD8. J Immunol. 2000;164(12):6100-6104. [17] Lim JH, Kim JS, Yoon IH, et al. Immunomodulation of delayed-type hypersensitivity responses by mesenchymal stem cells is associated with bystander T cell apoptosis in the draining lymph node. J Immunol. 2010;185(7):4022-4029. [18] Benvenuto F, Ferrari S, Gerdoni E, et al. Human mesenchymal stem cells promote survival of T cells in a quiescent state. Stem Cells. 2007;25(7):1753- 1760. [19] Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101(9):3722-3729. [20] Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838-3843. [21] Tse WT, Pendleton JD, Beyer WM, et al. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75(3):389-397. [22] Soleymaninejadian E, Pramanik K, Samadian E. Immunomodulatory properties of mesenchymal stem cells: cytokines and factors. Am J Reprod Immunol. 2012;67(1):1-8. [23] Augello A, Tasso R, Negrini SM, et al. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 2005;35(5):1482-1490. [24] Sheng H, Wang Y, Jin Y, et al. A critical role of IFNgamma in priming MSC-mediated suppression of T cell proliferation through up-regulation of B7-H1. Cell Res. 2008;18(8):846-857. [25] Zafranskaya M, Nizheharodava D, Yurkevich M, et al. PGE2 contributes to in vitro MSC-mediated inhibition of non-specific and antigen-specific T cell proliferation in MS patients. Scand J Immunol. 2013; 78(5):455-462. [26] De Miguel MP, Fuentes-Julián S, Blázquez-Martínez A, et al. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med. 2012;12(5):574-591. [27] Lysak D, Vlas T, Holubova M, et al. In vitro testing of immunosupressive effects of mesenchymal stromal cells on lymphocytes stimulated with alloantigens. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015;159(2):215-219. [28] Zhou Y, Day A, Haykal S, et al. Mesenchymal stromal cells augment CD4+ and CD8+ T-cell proliferation through a CCL2 pathway. Cytotherapy. 2013;15(10): 1195-1207. [29] Schena F, Gambini C, Gregorio A, et al. Interferon-γ-dependent inhibition of B cell activation by bone marrow-derived mesenchymal stem cells in a murine model of systemic lupus erythematosus. Arthritis Rheum. 2010;62(9):2776-2786. [30] Rosado MM, Bernardo ME, Scarsella M, et al. Inhibition of B-cell proliferation and antibody production by mesenchymal stromal cells is mediated by T cells. Stem Cells Dev. 2015;24(1):93-103. [31] Ren G, Zhang L, Zhao X, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2(2):141-150. [32] Rasmusson I, Le Blanc K, Sundberg B, et al. Mesenchymal stem cells stimulate antibody secretion in human B cells. Scand J Immunol. 2007;65(4): 336-343. [33] Traggiai E, Volpi S, Schena F, et al. Bone marrow-derived mesenchymal stem cells induce both polyclonal expansion and differentiation of B cells isolated from healthy donors and systemic lupus erythematosus patients. Stem Cells. 2008;26(2): 562-569. [34] Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107(1):367-372. [35] Okazaki T, Maeda A, Nishimura H, et al. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci U S A. 2001; 98(24):13866-13871. [36] Che N, Li X, Zhang L, et al. Impaired B cell inhibition by lupus bone marrow mesenchymal stem cells is caused by reduced CCL2 expression. J Immunol. 2014; 193(10): 5306-5314. [37] Che N, Li X, Zhou S, et al. Umbilical cord mesenchymal stem cells suppress B-cell proliferation and differentiation. Cell Immunol. 2012;274(1-2):46-53. [38] Spaggiari GM, Capobianco A, Abdelrazik H, et al. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111(3):1327-1333. [39] Sotiropoulou PA, Perez SA, Gritzapis AD, et al. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24(1):74-85. [40] Ryan JM, Barry F, Murphy JM, et al. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol. 2007;149(2):353-363. [41] Spaggiari GM, Capobianco A, Becchetti S, et al. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107(4):1484-1490. [42] Götherström C, Lundqvist A, Duprez IR, et al. Fetal and adult multipotent mesenchymal stromal cells are killed by different pathways. Cytotherapy. 2011;13(3): 269-278. [43] Yu Y, Wang Y, Feng M. Human leukocyte antigen-G1 inhibits natural killer cytotoxicity through blocking the activating signal transduction pathway and formation of activating immunologic synapse. Hum Immunol. 2008; 69(1):16-23. [44] Jiang XX, Zhang Y, Liu B, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105(10): 4120-4126. [45] Nauta AJ, Kruisselbrink AB, Lurvink E, et al. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte- derived dendritic cells. J Immunol. 2006;177(4): 2080-2087. [46] Spaggiari GM, Abdelrazik H, Becchetti F, et al. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood. 2009;113(26):6576-6583. [47] Yoo KH, Jang IK, Lee MW, et al. Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell Immunol. 2009;259(2):150-156. [48] Najar M, Raicevic G, Boufker HI, et al. Mesenchymal stromal cells use PGE2 to modulate activation and proliferation of lymphocyte subsets: Combined comparison of adipose tissue, Wharton's Jelly and bone marrow sources. Cell Immunol. 2010;264(2): 171-179. [49] Ribeiro A, Laranjeira P, Mendes S, et al. Mesenchymal stem cells from umbilical cord matrix, adipose tissue and bone marrow exhibit different capability to suppress peripheral blood B, natural killer and T cells. Stem Cell Res Ther. 2013;4(5):125. [50] Xishan Z, Baoxin H, Xinna Z, et al. Comparison of the effects of human adipose and bone marrow mesenchymal stem cells on T lymphocytes. Cell Biol Int. 2013;37(1):11-18. [51] Li X, Bai J, Ji X, et al. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int J Mol Med. 2014; 34(3):695-704. [52] Bochev I, Elmadjian G, Kyurkchiev D, et al. Mesenchymal stem cells from human bone marrow or adipose tissue differently modulate mitogen-stimulated B-cell immunoglobulin production in vitro. Cell Biol Int. 2008;32(4):384-393. [53] Yen BL, Chang CJ, Liu KJ, et al. Brief report--human embryonic stem cell-derived mesenchymal progenitors possess strong immunosuppressive effects toward natural killer cells as well as T lymphocytes. Stem Cells. 2009;27(2):451-456. [54] Ivanova-Todorova E, Bochev I, Mourdjeva M, et al. Adipose tissue-derived mesenchymal stem cells are more potent suppressors of dendritic cells differentiation compared to bone marrow-derived mesenchymal stem cells. Immunol Lett. 2009;126(1-2): 37-42. [55] Saeidi M, Masoud A, Shakiba Y, et al. Immunomodulatory effects of human umbilical cord Wharton's jelly-derived mesenchymal stem cells on differentiation, maturation and endocytosis of monocyte-derived dendritic cells. Iran J Allergy Asthma Immunol. 2013;12(1):37-49. [56] Yoo HS, Yi T, Cho YK, et al. Mesenchymal Stem Cell Lines Isolated by Different Isolation Methods Show Variations in the Regulation of Graft-versus-host Disease. Immune Netw. 2013;13(4):133-140. [57] Wu LW, Wang YL, Christensen JM, et al. Donor age negatively affects the immunoregulatory properties of both adipose and bone marrow derived mesenchymal stem cells. Transpl Immunol. 2014;30(4):122-127. [58] Najar M, Raicevic G, Fayyad-Kazan H, et al. Impact of different mesenchymal stromal cell types on T-cell activation, proliferation and migration. Int Immunopharmacol. 2013;15(4):693-702. [59] Yazid FB, Gnanasegaran N, Kunasekaran W, et al. Comparison of immunodulatory properties of dental pulp stem cells derived from healthy and inflamed teeth. Clin Oral Investig. 2014;18(9):2103-2112. [60] Wagner B, Henschler R. Fate of intravenously injected mesenchymal stem cells and significance for clinical application. Adv Biochem Eng Biotechnol. 2013;130: 19-37. [61] Li H, Jiang Y, Jiang X, et al. CCR7 guides migration of mesenchymal stem cell to secondary lymphoid organs: a novel approach to separate GvHD from GvL effect. Stem Cells. 2014;32(7):1890-1903. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [3] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [4] | Zhang Chao, Lü Xin. Heterotopic ossification after acetabular fracture fixation: risk factors, prevention and treatment progress [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1434-1439. |
| [5] | Zhou Jihui, Li Xinzhi, Zhou You, Huang Wei, Chen Wenyao. Multiple problems in the selection of implants for patellar fracture [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1440-1445. |
| [6] | Wang Debin, Bi Zhenggang. Related problems in anatomy mechanics, injury characteristics, fixed repair and three-dimensional technology application for olecranon fracture-dislocations [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1446-1451. |
| [7] | Ji Zhixiang, Lan Changgong. Polymorphism of urate transporter in gout and its correlation with gout treatment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1290-1298. |
| [8] | Yuan Mei, Zhang Xinxin, Guo Yisha, Bi Xia. Diagnostic potential of circulating microRNA in vascular cognitive impairment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1299-1304. |
| [9] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [10] | Shi Yangyang, Qin Yingfei, Wu Fuling, He Xiao, Zhang Xuejing. Pretreatment of placental mesenchymal stem cells to prevent bronchiolitis in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 991-995. |
| [11] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [12] | Fan Quanbao, Luo Huina, Wang Bingyun, Chen Shengfeng, Cui Lianxu, Jiang Wenkang, Zhao Mingming, Wang Jingjing, Luo Dongzhang, Chen Zhisheng, Bai Yinshan, Liu Canying, Zhang Hui. Biological characteristics of canine adipose-derived mesenchymal stem cells cultured in hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1002-1007. |
| [13] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [14] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [15] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||