Chinese Journal of Tissue Engineering Research ›› 2016, Vol. 20 ›› Issue (36): 5419-5425.doi: 10.3969/j.issn.2095-4344.2016.36.015
Previous Articles Next Articles
Methylprednisolone effects on the migration of endogenous neural stem cells after spinal cord injury
Qu Yi-ming, Li Bo, Wang Qun-bo, Shao Gao-hai, Lu Min-peng, Yu Yu, Liu Zuo-zhong, Cao Chun-feng
- Department of Spinal Surgery, Yongchuan Hospital of Chongqing Medical University, Chongqing 402160, China
-
Revised:2016-07-06Online:2016-09-02Published:2016-09-02 -
Contact:Li Bo, Master, Associate professor, Master’s supervisor, Department of Spinal Surgery, Yongchuan Hospital of Chongqing Medical University, Chongqing 402160, China -
About author:Qu Yi-ming, Master, Attending physician, Department of Spinal Surgery, Yongchuan Hospital of Chongqing Medical University, Chongqing 402160, China -
Supported by:a grant from Chongqing Health Department, No. 2012-2-170; a grant from the Science and Technology Committee of Yongchuan District of Chongqing, China, No. 2012BE5013
CLC Number:
Cite this article
Qu Yi-ming, Li Bo, Wang Qun-bo, Shao Gao-hai, Lu Min-peng, Yu Yu, Liu Zuo-zhong, Cao Chun-feng . Methylprednisolone effects on the migration of endogenous neural stem cells after spinal cord injury[J]. Chinese Journal of Tissue Engineering Research, 2016, 20(36): 5419-5425.
share this article
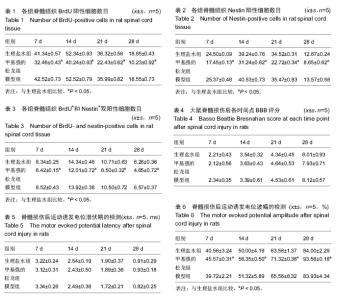
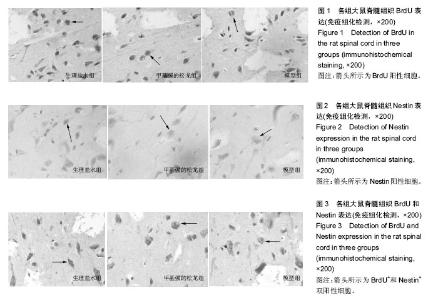
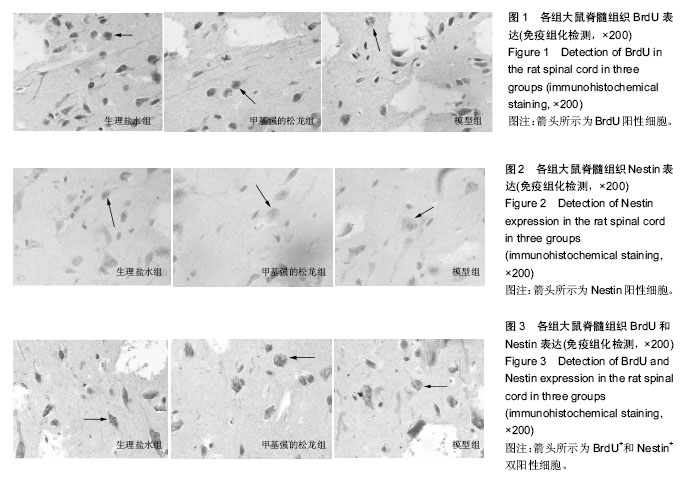
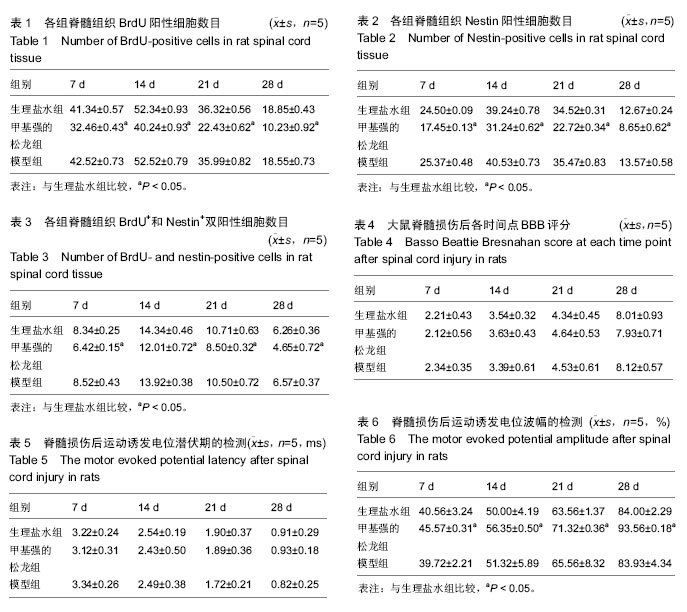
2.1 实验动物数量分析 75只SD大鼠均进入结果分析。 2.2 甲基强的松龙抑制损伤脊髓内BrdU染色的细胞数量 BrdU染色的细胞数量及位置可以用于检测内源神经干细胞的增殖及迁移。在同一倍数的镜下统计BrdU阳性细胞数目(图1)。在正常的大鼠脊髓切片中,大小均匀、圆形或椭圆形的细胞,即表明内源神经干细胞存在。各组BrdU细胞阳性数目见表1。统计分析显示,生理盐水组与模型组差异无显著性意义,甲基强的松龙组各时间点BrdU阳性细胞数均低于生理盐水组,差异有显著性意义(P < 0.05)。 2.3 甲基强的松龙抑制损伤脊髓内Nestin染色的细胞数量 3组各个时间段的Nestin标记的细胞数量与BrdU的变化相同(图2,表2),均在脊髓损伤后第14天达到最峰,随后降低。Nestin标记的神经干细胞主要集中脊髓损伤的部位。统计分析显示,在各个时间点甲基强的松龙组Nestin表达阳性细胞明显低于生理盐水组,模型组与生理盐水组差异无显著性意义,提示甲基强的松龙能够明显抑制脊髓损伤大鼠的神经干细胞的扩增及迁移。 2.4 甲基强的松龙抑制BrdU+和Nestin+双阳性的细胞数量 脊髓损伤后BrdU+和Nestin+双阳性细胞在14 d达到最高,随后降低,该细胞趋向于损伤部位,并且聚集于损伤部位周围(图3)。甲基强的松龙组与生理盐水组相比差异有显著性意义,模型组与生理盐水组差异无显著性意义,见表3。 2.5 甲基强的松龙对于后肢运动无显著性作用 利用BBB评分鉴定甲基强的松龙对于脊髓损伤后下肢活动的影响,各组之间比较,差异无显著性意义(P > 0.05),可见注射甲基强的松龙对于损伤脊髓大鼠后肢运动没有显著作用,见表4。 2.6 脊髓损伤后脊髓运动诱发电位潜伏期和波幅的改变 表5显示在各时间点各组大鼠运动诱发电位潜伏期,分析可见各组大鼠运动诱发电位潜伏期比较差异无显著性意义(P > 0.05),甲基强的松龙对于诱发电位潜伏期没有显著作用。表6显示在各时间点各组大鼠运动诱发电位波幅,可见甲基强的松龙组波幅显著比生理盐水组有所改善,即甲基强的松龙注射可以促进损伤脊髓神经传导的恢复。"
| [1] Cirillo G, Colangelo AM, De Luca C, et al. Modulation of Matrix Metalloproteinases Activity in the Ventral Horn of the Spinal Cord Re-stores Neuroglial Synaptic Homeostasis and Neurotrophic Support following Peripheral Nerve Injury. PLoS One. 2016;11(3):e0152750. [2] Trofimenko V, Hotaling JM. Fertility treatment in spinal cord injury and other neurologic disease. Transl Androl Urol. 2016;5(1):102-116. [3] Lemmon VP, Abeyruwan S, Visser U,Jet al. Facilitating transparency in spinal cord injury studies using data standards and ontologies. Neural Regen Res.2014; 9(1): 6-7. [4] Güzelküçük Ü, Demir Y, Kesikburun S, et al. Spinal cord injury resulting from gunshot wounds: a comparative study with non-gunshot causes. Spinal Cord. 2016 Mar 1. [Epub ahead of print] [5] Schwab JM, Chiang N, Arita M, et al. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447(7146):869-874. [6] Forner S, Martini AC, de Andrade EL, et al. Neuropathic pain induced by spinal cord injury: Role of endothelin ETA and ETB receptors. Neurosci Lett. 2016;617:14-21. [7] Yeng CH, Chen PJ, Chang HK, et al. Attenuating spinal cord injury by conditioned medium from human umbilical cord blood-derived CD34? cells in rats. Taiwan J Obstet Gynecol. 2016;55(1):85-93. [8] Dong Y, Miao L, Hei L, et al. Neuroprotective effects and impact on caspase-12 expression of tauroursodeoxycholic acid after acute spinal cord injury in rats. Int J Clin Exp Pathol. 2015;8(12): 15871-15878. [9] Knoller N, Auerbach G, Fulga V, et al. Clinical experience using incubated autologous macrophages as a treatment for complete spinal cord injury: phase I study results. J Neurosurg Spine. 2005;3(3):173-181. [10] Dong YZ,Yang LB,Yang L,et al.Transplantation of neurotrophin-3-transfected bone marrow mesenchymal stem cells for the repair of spinal cord injury. Neural Regen Res.2014;9(16): 1520-1524. [11] Erlandsson A, Lin CH, Yu F, et al. Immunosuppression promotes endogenous neural stem and progenitor cell migration and tissue regeneration after ischemic injury. Exp Neurol. 2011;230(1):48-57. [12] Bartholdi D, Schwab ME. Expression of pro-inflammatory cytokine and chemokine mRNA upon experimental spinal cord injury in mouse: an in situ hybridization study. Eur J Neurosci. 1997;9(7):1422- 1438. [13] Mao D, Yao X, Feng G, et al. Skin-derived precursor cells promote angiogenesis and stimulate proliferation of endogenous neural stem cells after cerebral infarction. Biomed Res Int. 2015;2015:945846. [14] Thored P, Arvidsson A, Cacci E, et al. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24(3):739-747. [15] Horky LL, Galimi F, Gage FH, et al. Fate of endogenous stem/progenitor cells following spinal cord injury. J Comp Neurol. 2006;498(4):525-538. [16] Goldman S. Stem and progenitor cell-based therapy of the human central nervous system. Nat Biotechnol. 2005;23(7):862-871. [17] Hohlfeld R, Kerschensteiner M, Meinl E. Dual role of inflammation in CNS disease. Neurology. 2007;68 (22 Suppl 3):S58-63. [18] Yacoubian S, Serhan CN. New endogenous anti-inflammatory and proresolving lipid mediators: implications for rheumatic diseases. Nat Clin Pract Rheumatol. 2007;3(10):570-579. [19] de Araújo RF Jr, Reinaldo MP, Brito GA, et al. Olmesartan decreased levels of IL-1β and TNF-α, down-regulated MMP-2, MMP-9, COX-2, RANK/RANKL and up-regulated SOCs-1 in an intestinal mucositis model. PLoS One. 2014;9(12):e114923. [20] Sakthivel KM, Guruvayoorappan C. Acacia ferruginea inhibits tumor progression by regulating inflammatory mediators-(TNF-a, iNOS, COX-2, IL-1β, IL-6, IFN-γ, IL-2, GM-CSF) and pro-angiogenic growth factor- VEGF. Asian Pac J Cancer Prev. 2013;14(6):3909-3919. [21] Aharoni R, Saada R, Eilam R, et al. Oral treatment with laquinimod augments regulatory T-cells and brain-derived neurotrophic factor expression and reduces injury in the CNS of mice with experimental autoimmune encephalomyelitis. J Neuroimmunol. 2012;251(1-2):14-24. [22] Stirling DP, Liu S, Kubes P, et al. Depletion of Ly6G/Gr-1 leukocytes after spinal cord injury in mice alters wound healing and worsens neurological outcome. J Neurosci. 2009;29(3):753-764. [23] Kawasaki Y, Zhang L, Cheng JK, et al. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28(20):5189-5194. [24] He X, Shu J, Xu L, et al. Inhibitory effect of Astragalus polysaccharides on lipopolysaccharide-induced TNF-a and IL-1β production in THP-1 cells. Molecules. 2012; 17(3):3155-3164. [25] Boato F, Rosenberger K, Nelissen S, et al. Absence of IL-1β positively affects neurological outcome, lesion development and axonal plasticity after spinal cord injury. J Neuroinflammation. 2013;10:6. [26] Hassanshahi G, Amin M, Shunmugavel A, et al. Temporal expression profile of CXC chemokines in serum of patients with spinal cord injury. Neurochem Int. 2013;63(5):363-367. [27] De Filippis L, Südhof TC, Pang ZP. Harness the power of endogenous neural stem cells by biomaterials to treat spinal cord injury. Sci China Life Sci. 2015;58(11): 1167-1168. [28] Lytle JM, Wrathall JR. Glial cell loss, proliferation and replacement in the contused murine spinal cord. Eur J Neurosci. 2007;25(6):1711-1724. [29] Mladinic M, Nistri A. Dynamic expression of ATF3 as a novel tool to study activation and migration of endogenous spinal stem cells and their role in neural repair. Neural Regen Res. 2015;10(5):713-714. [30] Durak H, Atmaca S, Karayi?it MÖ, et al. Effect of Mitomycin C on bFGF, TGF-β1, KGF-1 Expressions after Myringotomy: An Animal Study. J Int Adv Otol. 2015;11(3):188-191. [31] Hall ED. Methylprednisolone for the Treatment of Patients with Acute Spinal Cord Injuries: A Propensity Score-Matched Cohort Study from a Canadian Multi-Center Spinal Cord Injury Registry. J Neurotrauma. 2016;33(10):972-974. [32] Wang W, Wang P, Li S, et al. Methylprednisolone inhibits the proliferation and affects the differentiation of rat spinal cord-derived neural progenitor cells cultured in low oxygen conditions by inhibiting HIF-1α and Hes1 in vitro. Int J Mol Med. 2014;34(3):788-795. [33] Jing YH, Hou YP, Song YF, et al. Methylprednisolone improves the survival of new neurons following transient cerebral ischemia in rats.Acta Neurobiol Exp (Wars). 2012;72(3):240-252. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Guan Qian, Luan Zuo, Ye Dou, Yang Yinxiang, Wang Zhaoyan, Wang Qian, Yao Ruiqin. Morphological changes in human oligodendrocyte progenitor cells during passage [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1045-1049. |
| [3] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [4] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [5] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [6] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [7] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [8] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [9] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [10] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [11] | Xing Hao, Zhang Yonghong, Wang Dong. Advantages and disadvantages of repairing large-segment bone defect [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 426-430. |
| [12] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| [13] | Wang Hao, Chen Mingxue, Li Junkang, Luo Xujiang, Peng Liqing, Li Huo, Huang Bo, Tian Guangzhao, Liu Shuyun, Sui Xiang, Huang Jingxiang, Guo Quanyi, Lu Xiaobo. Decellularized porcine skin matrix for tissue-engineered meniscus scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3473-3478. |
| [14] | Mo Jianling, He Shaoru, Feng Bowen, Jian Minqiao, Zhang Xiaohui, Liu Caisheng, Liang Yijing, Liu Yumei, Chen Liang, Zhou Haiyu, Liu Yanhui. Forming prevascularized cell sheets and the expression of angiogenesis-related factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3479-3486. |
| [15] | Liu Chang, Li Datong, Liu Yuan, Kong Lingbo, Guo Rui, Yang Lixue, Hao Dingjun, He Baorong. Poor efficacy after vertebral augmentation surgery of acute symptomatic thoracolumbar osteoporotic compression fracture: relationship with bone cement, bone mineral density, and adjacent fractures [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3510-3516. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||