Chinese Journal of Tissue Engineering Research ›› 2016, Vol. 20 ›› Issue (30): 4524-4531.doi: 10.3969/j.issn.2095-4344.2016.30.017
Previous Articles Next Articles
Morpholine-2, 5-dione and its derivatives: the valuable biodegradable polymer materials
Zhou Qun-hua1, Yang Li-qun2, Zhang Wei2, Li Miao2
- 1Zhengzhou Shuqing Medical College, Zhengzhou 450064, Henan Province, China; 2Liaoning Provincial Family Planning Research Institute, Key Laboratory of Reproductive Health and Medical Genetics, National Health and Family Planning Commission, Shenyang 110031, Liaoning Province, China
-
Received:2016-05-30Online:2016-07-15Published:2016-07-15 -
Contact:Yang Li-qun, Associate researcher, Liaoning Provincial Family Planning Research Institute, Key Laboratory of Reproductive Health and Medical Genetics, National Health and Family Planning Commission, Shenyang 110031, Liaoning Province, China -
About author:Zhou Qun-hua, Lecturer, Zhengzhou Shuqing Medical College, Zhengzhou 450064, Henan Province, China -
Supported by:the National Natural Science Foundation of China, No. 51503093; the Science Public Research Foundation of Liaoning Province, No. 2015001007; the Doctoral Scientific Research Foundation of Liaoning Province, No. 201501116; the Scientific Research Project of Shenyang, China, No. F16-205-1-37
CLC Number:
Cite this article
Zhou Qun-hua, Yang Li-qun, Zhang Wei, Li Miao. Morpholine-2, 5-dione and its derivatives: the valuable biodegradable polymer materials[J]. Chinese Journal of Tissue Engineering Research, 2016, 20(30): 4524-4531.
share this article
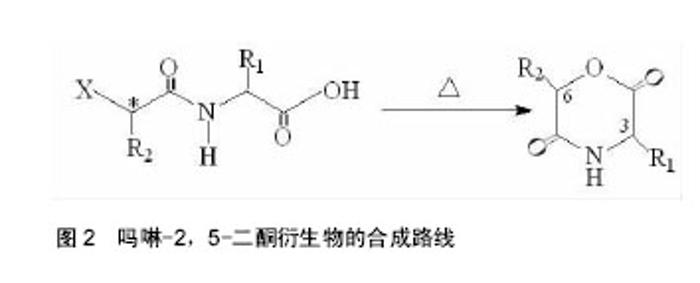
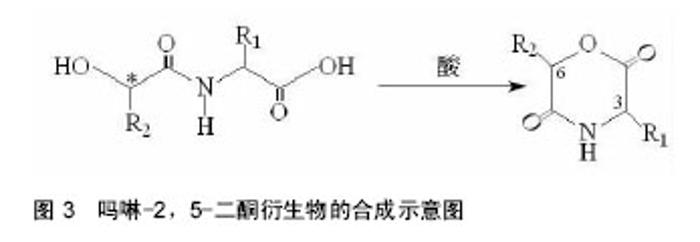
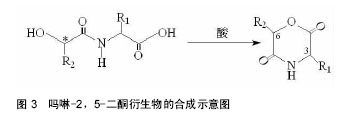
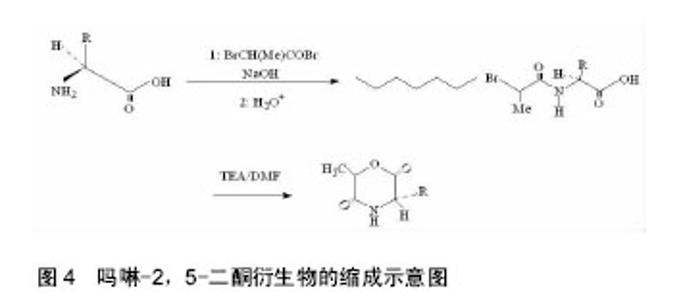
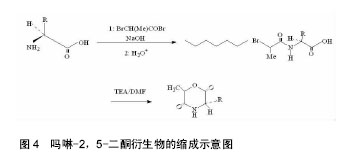
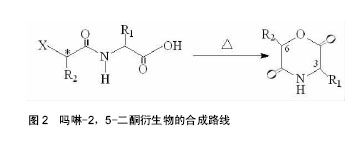
2.1 吗啉-2,5-二酮及其衍生物单体的合成 寻找到优异的吗啉-2,5-二酮及其衍生物的单体的合成方法是合成其聚合物的首要条件,近年来,很多研究人员通过各种途径对吗啉-2,5-二酮及其衍生物单体的合成进行了大量的研究工作。根据不同的化学反应路径,吗啉-2,5-二酮及其衍生物的合成方法大致可分为以下3种:①Chadwick等通过加热N-(2-溴丙酰基)-甘氨酸钠,有效的合成了3,6位烷基取代的吗啉-2,5-二酮衍生物,合成路线见图2。Glowaky等[15]则报道了N-(α-卤酰基)-α-氨基酸在二甲基甲酰胺稀溶液里的成环反应,得到相同产物,1990年,Fung等[16]以相同方法,通过改变原料氨基酸的种类而制备出多种吗 啉-2,5-二酮的衍生物。Feijen等[17]利用这种方法首次合成了(3S,6RS)-3-(苄氧羰基甲基)-6-甲基-吗啉- 2,5-二酮、(3S,6RS)-3-[4-(苄氧羰基氨基)丁基]-6-甲基-吗啉-2,5-二酮、(3S,6RS)-3-对甲氧苄基硫代羟甲基-6-甲基-吗啉-2,5-二酮等带有官能团侧基的吗啉-2,5-二酮衍生物。冯新德等[18-19]探索了在极稀溶液里成环的方法合成带有官能团侧基的吗啉-2,5-二酮衍生物,获得了长足的进展。此种方法可通过反应物的酰化反应直接制得,方法简单,并且不需要苛刻的条件,产率较高,约为25%。但是,缺点也尤为突出:在成环过程中,靠近卤素的手性中心发生了外消旋作用,造成提纯困难,难以得到纯的光学左旋体或右旋体,而且反应时间较长,效率较低。②Hartwig等[20]第1次用酸催化环合了N-(α-羟酸基)-α-氨基酸,制备了一系列的3,6位烷基取代的吗啉-2,5-二酮衍生物。这是一个分子内的酯化反应,在反应中,反应位置不在手性碳原子上,所以手性中心没有涉及,不会发生消旋反应,合成路线见图3。而Nakamura等[21]在甲苯溶液中,使N-(α-羟基)-α-氨基酸酯发生酯交换反应,来合成吗啉-2,5-二酮单体,但反应温度却在150-350 ℃之间。由于此种反应方法较高的温度,而极易导致 α-氨基酸酯发生部分消旋现象。此后,Hocker等通过各种反应途径探索了单体旋光性的保持及关环反应,并做了较大的改进,使反应条件更温和,环单体保持原构型。利用N-(α-羟酸基)-α-氨基酸的环化来制备吗啉-2,5-二酮衍生物时常见的3种反应条件,即:甲苯磺酸或甲磺酸作为催化剂,产率较高;羧酸作为环化剂,产率不高;高温减压条件下,副产物较少。③有研究等用O-(α-氨基酰基)-α-氨基酸进行分子内的缩合反应得到吗啉-2,5-二酮衍生物,合成路线见图4。Nissen等[22]以O-(α-氨基酰基)-α-氨基酸为原料通过此种方法合成出了3-苄氧甲基和 3-对甲氧基硫代羟甲基的6-甲基-吗啉-2,5-二酮单体,但除主产物外,还伴有低聚物生成。它的优点在于可以得到光学纯的产物,无消旋,缺点是在合成过程中使用了多步的活化、保护、去保护步骤、产率较低(低于10%)。通过以上3种常用方法的比较,不难发现这些方法得到的产物有的产率低,有的不纯,有的需要较高的条件,有的是反应过程复杂。所以,一种较完善的合成吗啉-2,5-二酮及其衍生物单体的方法迫切需要探索。"
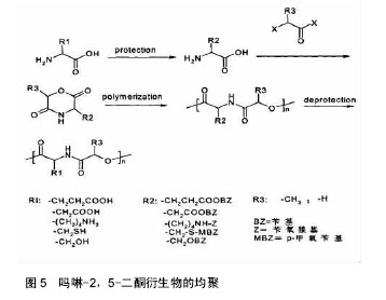
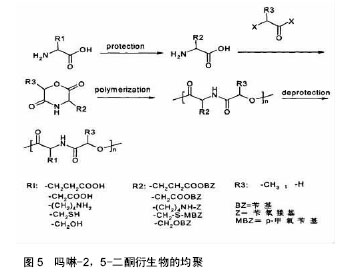
2.2 吗啉-2,5-二酮及其衍生物的聚合 2.2.1 吗啉-2,5-二酮及其衍生物的均聚 吗啉-2,5-二酮及其衍生物的成环、均聚反应过程如图5所示,由图5可以看出,含有第三官能团的α-氨基酸和α-羟基酸作为原料合成吗啉-2,5-二酮及其衍生物时,容易发生严重的副反应,而造成产物不纯,损失产量,产品性能不好,所以,在成环、均聚反应过程中需要对第三官能团加以保护。早在1985年,Helder等[23]在 130 ℃的反应条件下,以辛酸亚锡引发下开环自聚,得到了较高分子量的聚合产物。1993年,Ouchi等[24]首次利用β-苄基-L-天冬氨酸和羟基乙酸合成了其环状二聚体,经过开环聚合和改性,可得到具有良好的体外降解和酶降解特性的聚合物。日本的研究小组对3位、6位有取代基的吗啉-2,5-二酮及其衍生物进行均聚,却发现需要较高的条件,同时得到的产物分子量低,性质不稳定等。原因可能是烷基取代基具有较大的位租,基团难以进攻母体。后来,世界各国的多个课题组又对吗啉-2,5-二酮及其衍生物的均聚物进行大量研究,都发现所得产物溶解性差,有较高的玻璃化转变温度,同时结晶性也差,缺乏良好的应用前景。因此,需要在吗啉-2,5-二酮及其衍生物的聚合过程中引入其他物质,进行改性实验。 2.2.2 吗啉-2,5-二酮及其衍生物的共聚 吗啉-2,5-二酮及其衍生物的均聚物由于性质上的缺憾,而目前对生物医用材料要求越来越高,所以对其改性势在必行。通过把α-羟基酸和α-氨基酸制成六元环内酯酰胺,然后开环聚合,引入己内酯、丙交酯等物质,而得到α-羟基酸和α-氨基酸的交替或嵌段共聚物,由于这些聚合物都包含酯键和酰胺键,所以其生物降解性、生物相容性等性质比起均聚物优越[25]。而且合成步骤简单,反应条件温和,因此此种方法具有更加广阔的前景,应用更加广泛。近年来国内外科研人员合成出了一系列的α-羟基酸和α-氨基酸共聚物,并对其性能进行了研究。 吗啉-2,5-二酮与己内酯共聚:聚己内酯由于具有良好的生物降解性、生物相容性及药物透过性,而被广泛应用于药物控释、医疗器械等领域。然而聚己内酯又因强度不高、降解速率缓慢、水溶性差且无功能基团,使其应用领域受限[26]。因而,对聚己内酯进行改性非常必要,常采用的方法是共聚、接枝和共混等。"
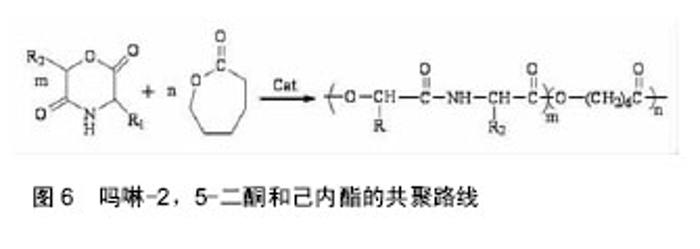
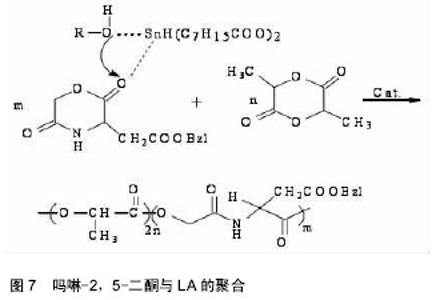
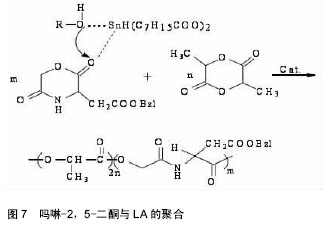
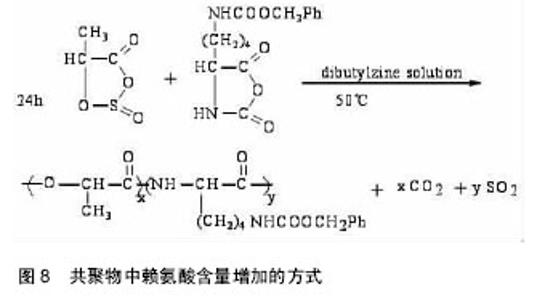
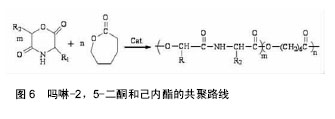
吗啉二酮与ε-已内酯 共聚反应如图6所示。 带有官能团的吗啉-2,5-二酮与内酯的共聚能力非常强大,其大多数都能与内酯(如己内酯、丙交脂)发生共聚反应,产物具有良好的细胞亲和性和一定的亲水性。多种氨基酸的吗啉-2,5-二酮衍生物都能与ε-已内酯进行共聚,如丝氨酸、天门冬氨酸;Intveld等[27]报道了cyclo[LA-L-Asp(BZ)],cyelo[LA-L- Lys(Z)], cyelo[LA-L-Cys(MBz)]分别与丙交酯、己内醋的共聚反应;Wang等[28]用(3S)-[(苄氧羰基)-甲基]-吗啉-2,5-二酮和ε-已内酯开环共聚,其聚合物的降解产物是乙醇酸和天冬氨酸,无细胞毒性且可参与体内的代谢反应;2011年,Batting等[29]合成了含有聚己内酯和聚异丁基吗啉-2,5-二酮链段的多嵌段共聚物,其具备良好的弹性性能和生物降解性能,有望得到广泛的应用。 吗啉二酮与丙交脂共聚:聚丙交脂是一种重要的生物可降解聚合物,具有良好的生物相容性、生物降解性、生物可吸收性和一定的力学性能,从而被广泛应用于手术缝合线、药物控释载体、骨固定与组织工程支架材料等生物医学领域[30-31]。然而单纯的聚丙交酯也具有不可忽视的缺点,即具有高的结晶度、疏水性、降解速率慢及力学韧性较差[32-33]。所以为了增加聚丙交酯在生物医学中的广泛应用,需要对聚丙交酯进行改性。 早期,很多学者利用烷基取代的吗啉-2,5-二酮与丙交酯共聚,对其分子质量、降解性能及黏度进行了检测,却发现性能不理想,原因可能是丙交酯的聚合活性比吗啉-2,5-二酮大。2002年,Hiroyuki等[34]把3,6-二甲基-吗啉-2,5-二酮和L-LA进行了共聚,共聚产物虽然具有较高的抗拉强度、降解速度,但是对聚丙交酯低的伸长比没有改善。后来,随着越来越多的研究发现,带有官能团的吗啉-2,5-二酮与内酯的共聚物具有更优的性能。 In´t Veld等[35]报道了含有L-天冬氨酸、L-赖氨酸等氨基酸的吗啉-2,5 -二酮分别与丙交酯共聚反应。Guan等[36]以带有官能团的吗啉二酮衍生物3s-3-[(苄氧羰基)-乙基]吗啉二酮与丙交酯在辛酸亚锡催化下,合成出ABA型高分子,并进行了表征,发现具有高分子量、较好的降解性能。Ouehi等[37]研究出聚LLA-天冬氨酸与聚LLA-赖氨酸共聚物,发现了共聚物的纯降解速率与酶解速率都比聚LLA降解速率快得多。此外,吗啉-2,5 -二酮共聚物也可以作为药物载体,使药物缓慢释放,如Vavia等合成了聚[乳酸-(乙醇酸-亮氨酸)]共聚物,作为包埋药物环丙沙星的聚合物颗粒。Wang等[38]首先合成了6位上无取代基的β-苄酯保护的天冬氨酸的吗啉二酮,随后使其成功地进行了与丙交酯的共聚反应,如图7。得到的共聚物经催化脱保护基后,可用作多肽类药物载体[39-40]。吗啉二酮与丙交酯、已内酯和氨基酸等的开环聚合是制备优异的可生物降解材料的一种很好的方法[41],但由于吗啉二酮的活性比己内酯等单体要低,而造成引入的氨基酸的量偏低,会受到投料的影响。随着吗啉二酮的量的增加,聚合物中氨基酸的量也增加,但是聚合物的性质受到了影响,致使分子量和转化率都降低了。刘育等用图8所示的两种单体聚合,使得引入赖氨酸的量达到了10%[42]。 2.3 吗啉二酮聚合引发剂/催化剂体系的研究 因为吗 啉-2,5-二酮衍生物的结构与环状内酯的结构相似,所以用于环状内酯开环聚合的引发剂/催化剂体系也同样可以用于吗啉-2,5-二酮衍生物的开环聚合,目前较为常用的方法是金属化合物催化聚合(如辛酸亚锡)、绿色催化剂酶催化聚合。 2.3.1 金属化合物催化聚合 金属化合物催化聚合(如辛酸亚锡)的机制被众多学者广泛的研究。目前得到认可的是二次插入机制,即辛酸亚锡起催化剂作用,而水和含羟基化合物作为引发剂,共同催化吗啉-2,5-二酮衍生物单体的开环聚合[43]。1998年,Volker等[44]用辛酸亚锡催化(3s,6s)-3-异丙基-6-甲基-吗琳-2,5-二酮自聚,得出催化机制同样是二次插入机 制,产物只有轻微的消旋,而且消旋现象仅仅发生在α-羟基酸的手性碳上。辛酸亚锡可使单体具有高的转化率,使产物具有低的消旋化。2001年,Krleheldorf 等[45]把2,2-二丁基-2-锡-1,3-二氧环丁烷(DSDOP)用于引发3,6位烷基取代吗啉-2,5一二酮衍生物的开环聚合,得到了高分子量的聚合物。除了金属锡作为催化剂外,还有含锌的催化剂。金属化合物催化聚合效率高,产量大,而且可以制得较纯的产品,但缺点也很明显,大部分的金属化合物都有毒性,给环境带来污染,影响人体的健康。 2.3.2 酶催化聚合 近年来,随着人们对环境保护意识的提高,为了避免对环境造成的污染,具有高度催化效率的酶催化剂,因为无污染,较好的生物相容性,越来越引起学者的关注。酶催化剂被广泛应用于高分子材料的降解、医药行业、日常生活、工业及生命保健等领域[46]。Feng等[47]利用不同的生物酶催化3,6位烷基取代吗啉-2,5-二酮衍生物开环聚合,研究了所得产物的转化率、数均分子量的异同等[48],并且发现聚合所需时间短,条件温和,不需要较高的温度,容易实现。同样,Yakai等[49-50]也用同种吗啉-2,5-二酮衍生物在不同酶催化下进行聚合,得到了性质较好的产物,但却发现聚合物中a-氨基酸和a-羟基酸部分的手性碳都发生了外消旋作用,而难以得到纯产品。 酶催化聚合有着金属催化聚合不可比拟的优势:无污染,生物相容性好,催化效率高,所合成聚合物的分子量分布很窄(1.04-1.16),分子量高,可达2万;其缺点是:3位和6位的碳都发生外消旋现象,造成产物不纯。 2.4 吗啉二酮的应用 合成的吗啉-2,5-二酮的自聚物及共聚物种类较多,合成方法也日趋成熟,经过系列实验证明其聚合物生物相容性和可生物降解性较好,近年越来越引起人们的重视。其应用主要有两大方面:一方面可作为药物控释载体,应用于医药领域;另一方面可应用于缝合线、人工关节,人工脏器等人工材料。 Ouchi[51]小组用表面具有官能团的吗啉-2,5-二酮,丙交酯和羟基乙酸与天冬氨酸的共聚物poly(LA-(Glc-L-Asp))和丙交酯和羟基乙酸与赖氨酸的共聚物poly(LA-(Glc–L-Lys)),通过油/水溶剂挥发法,分别做成表面悬挂有羧基和氨基的微球。并且成功地把模型药物1-萘磺酸接到了poly(LA-(Glc–L-Lys))的微球表面。通过研究微球的载药和控制释放能力,发现在相同条件下poly(LA-(Glc–L-Lys))微球的载药量比聚乳酸微球大,释药速度比聚乳酸微球慢。这就解决了用聚乳酸制成的微球表面因为没有官能团,不能通过化学键键合药物,缓释过程难以控制的问题[52]。 Ouchi等[53]首先制备了聚丙交酯和L-赖氨酸的共聚物poly(LA-co-L-Lys)。然后将小肽RGD(精氨酸-甘氨酸-天冬氨酸)接枝到聚合物表面赖氨酸的氨基上,接枝后的聚合物表面的细胞吸附能力有了很大提高。Hoffman等[54]制得丙烯酸酯化的poly(L-LA-co-(Glc- alt-L-Ser))和poly(CL-co-(Glc-alt-L-Ser)),光引发交联,制得聚合物网络和微球,并通过在交联过程中加入亲水性的物质,改善了交联网络在水中的溶胀比。这在组织工程支架材料的研究发展中有着很大的作用。 "
| [1] Pego AP,Poot AA,Grijpma DW,et al.Copolymers of trimethylene carbonate and ε-caprolactone for porous nerve guides: Synthesis and properties. Biomater Sci Polym Ed.2001;12:35-53.
[2] [Schappacher M,Fabre T,Mingotaud AF,et al.Study of a (trimethylenecarbonate-co-ε-caprolactone) polymer—Part 1: preparation of a new nerve guide through controlled random copolymerization using rare earth catalysts Biomaterials. 2001;22:2849-2855.
[3] Marina SP,Kapil A,Andrew O,et al.Polymer carriers for drug delivery in tissue engineering.Adv Drug Delivery Rev.2007;59:187-206.
[4] Seal BL,Otero TC,Panitch A,et al.Polymeric biomaterials for tissue and organ regeneration Mater Sci Eng R.2001;34:147-230.
[5] Boland ED,Pawlowski KJ,Barnes CP,et al. Electrospinning of bioresorbable polymers for tissue engineering scaffolds.Polymeric Nanofibers. 2006;918: 188-204.
[6] Mathiowitz E,Jacob JS,Jong YS,et al.Biologically erodable microspheres as potential oral drug delivery systems.Nature.1997;386(6623): 410-414.
[7] Zhang Z,Foks MA,Grijpma DW,et al.Synthesis and drug release behavior of poly (trimethylene carbonate)–poly (ethylene glycol)–poly (trimethylene carbonate) nanoparticles.J Controll Release. 2005; 101:392-394.
[8] Zhang Y,Zhuo RX.Synthesis and drug release behavior of poly (trimethylene carbonate)–poly (ethylene glycol)–poly (trimethylene carbonate) nanoparticles.Biomaterials.2005;26:2089-2094.
[9] 王身国.可生物降解的高分子类型、合成和应用[J].化学通报,1997,64(2):45.
[10] 黄发荣,陈涛,沈学宁.高分子材料的循环利用[M].北京:化学工业出版社,2000.
[11] 于丽,冯亚凯,石长灿,等.吗啉-2,5-二酮衍生物及其聚合物的研究进展[J].高分子通报,2015,28(3):18-25.
[12] Xie ZG,Lu TC,Chen XS,et al.Effects of Gel Concentration, Human Fibronectin, and Cation Supplement on the Tissue-Engineered Cartilage. Biomed Mater Res A.2007;(1):238-245.
[13] Fonseca AC,Gil MH,Simoes PN.Biodegradable poly(ester amide)s-A remarkable opportunity for the biomedical area: Review on the synthesis, characterization and applications.Prog Polym Sci. 2014;39(7):1291-1311.
[14] Fonseca AC,Serra AC,Coelho JFJ,et al.Novel poly(ester amide)s from glycine and L-lactic acid by an easy and cost-effective synthesis. Polym Int. 2013; 62(5):736-743.
[15] Glowaky RC,Fung FM.Bioabsorbable polydepsipeptides, their preparation and use. European patent.322154[p].1989-06-28.
[16] Fung FN,Glowaky RC.Bioabsorbable polydepsipeptide, preparation and use thereof.USP, 4916209,1990. 4.10.
[17] In't Veld PJ,Dijkstra PJ,Feijen J.Synthesis of biodegradable polyesteramides with pendant functional groups.Macromol Chem.1992;193:2713.
[18] Wang D,Feng XD.Synthesis of poly(glycolic acid-alt-l-aspartic acid) from a Morpholine-2,5-dione Derivative.Macromolecules.1997;30:5688.
[19] John G,Tsuda S,Morita M.Synthesis and modification of new biodegradable copolymers:Serine/glycolic acid based copolymers.Polym Sci Part A Polym Chem.1997;35:1901.
[20] Hartwig W,Schollkopf U.Asymmetrische Synthesen über heterocyclische Zwischenstufen, XVI. Enantioselektive Synthese von α-Alkyl-α- phenylglycinen durch Alkylieren von an C-6 chiral substituierten 3,6-Dihydro-3- phenyl- 2H-1, 4-oxazin-2-onen.Liebigs Ann Chem.1982:1952.
[21] Nakamura CE,Cosimo RD,Moran JR.Process for the preparation of 3-6-substituted 2,5-morpholinediones, US Patent.5466801,1995-04-10
[22] Nissen D,Gilon C,Goodmann M.Polydepsipeptides, 4 synthesis of thealternating polydepsipeptides poly(Ala-Lac) and poly(Val-Lac).Macromol Chem Phys. 1975;1(1):23-53.
[23] Helder J,Kohn FE,Sato S,et al.Synthesis of poly[oxyethylidene carbonylimino(2-oxoethylene)] [poly(glycine-D,L-lactic acid)] by ring openingpolymerization.Macromol Chem Phys Rapid Comm,1985;6(1):9-14.
[24] Ouchi T,Shiratani M,Jinno M,et al.Synthesis of poly[(glycolic acid)-alt-(L-aspartic acid)] and its biodegradation behavior in vitro. Macromol Rapid Comm.1993;14(12):825-831.
[25] In’t Veld PJA,Dijkstra PJ,van Lochem JH,et al.Synthesis of alternating polydepsipeptides by ring-opening polymerization of morpholine-2,5-dione derivatives Macromol Chem Phys. 1990;191(8):1813-1825.
[26] Zhang HW,Bei JZ,Wang SG.Preparation and drug release behaviors of 5-fluorouracil loaded poly (glycolide-co-lactide-co-caprolactone) nanoparticles. Appl Polym Sci.2007;106(6):3757-3767.
[27] In’t Veld PJA,Dijkstra PJ.Synthesis of biodegradable poly-esteramides with pendant functional groups. Makromolekulare Chemie. 1992;193:2713-2730.
[28] Wang D,Feng XD.Copolymerization of ε-Caprolactone with(3S)-3-[(Benzyloxycarbonyl) methyl] morpholine-2,5-dione and the 13C NMR sequence analysis of the copolymer. Macromolecules. 1998; 31(12):3824-3831.
[29] Batting A,Hiebl B,Feng Y.Biological evaluation of degradable, stimuli-sensitive multiblock copolymers having polydepsipeptide- and poly(?-caprolactone) segments in vitro. Clin Hemorheol Micro. 2011;48(1): 161-172.
[30] 王珍萍,周涛,周科朝.丙交酯制备工艺的研究现状[J].材料导报,2006,20(8):52-54.
[31] Hyon SH,Khosrow J,Yoshito I.Synthesis of polylactides with different molecular weights. Biomaterials. 1997; 18(22):1503-1508.
[32] Anders SE,Mikael S.Properties of lactic acid based polymers and their correlation with composition.Prog Polym Sci.2002;27:1123-1163.
[33] Rasal RM,Janorkarc AV,Hirt DE.Poly(lactic acid) modifications.Prog Polym Sci.2010;35:338-356.
[34] Hiroyuki S,Aki T.Highly biodegradable copolymers composed of chiral depsipeptide and L-lactide units with favorable physical properties.J Polym Sci Part A Polym Chem.2002;40(3):302-316.
[35] In´t Veld PJA, Ye W.Copolymerization of ε-caprolactone and morpholine-2 , 5-dionederivatives.Makromol Chem. 1992;193:1927-1942.
[36] Guan H,Xie Z,Zhang P,et al.Synthesis and characterization of biodegradable amphiphilic triblock copolymers containing l-Gluta mic acid units. Biomacromolecules.2005;6(4):1954-1960.
[37] Ouchi T,Nozaki T,Ishikawa A,et al.Synthesis and enzymatic hydrolys is of lactic acid-depsipeptid ecopolymers with functionalized pendangr oups.J Polym Sci Part A Polym Chem.1997;35:377-383.
[38] Wang D,Feng XD.Synthesis of Poly(glycolic acid-alt-l-aspartic acid) from a morpholine-2,5-dione derivative.Macromolecules. 1997;30(19):5688.
[39] [Deng X,Yao J,Yuan M,et al.Synthesis of poly[(glycolic acid)-alt-(L-glutamic acid)]and poly{(lactic acid)-co-[(glycolic acid)-alt-(L-glutamic acid)].Macromol Chem Phys.2000;201(17):2371-2376.
[40] Hans RK,Ingrid K.Transesterification of poly (Llactone) with poly (glycolide), poly (β-propiolactone), and poly (ε-caprolactone).Maccromol Sci Chem.1987;24(11):1345.
[41] 王俊凤,张军,张学龙,等.聚乳酸合成的研究进展[J].化工时刊,2007,21(6):51-56.
[42] Feng YK,Klee D, Keul H,et al.Synthesis and characterization of new blockcopolymers with poly(ethyleneoxide) andpoly[3(S)-sec- butylmorpholine- 2,5-dione]sequences. Macromolecular Bioscience. 2001;1(1):30-39.
[43] Krieheldorf HR,Kreiser SI,Boetteher C.Polylactones: 31. Sn(II)octoate-initiated polymerization of L-Lactide: a mechanistic study.Polymer.1995;36(6):1253-1259.
[44] Volker J,Helmut K,Hartwig H.Polylnerization of (3S,6S)-3-isopropyl-6-methyl-2,5-morphoLinedione with tin oetoate and tin acetylaeetonate.Maeromol Chem Phys.1998;199(5):835-843.
[45] Krleheldorf HR,Hauser K.Polylaetones,45. Homo-and CoPolymerizatio-nsof 3-Methylporpholine-2,5-dione Initiated with a Cyclic Tin Alkoxide.Maeromol Chem Phys.2001;202(7):1219-1226.
[46] Feng YK,Klee D,Keul H,et al.Synthesis and characterization of new block copolymers with poly(ethylene oxide) and poly[3(S)-sec- butylmorpholine-2,5-dione]sequences, Macromolecular. Bioscience.2001;1(1):30-39.
[47] Feng YK,Klee D,Hocker H.Synthesis of poly[(lactic acid)-alt-or co-((S)-aspartic acid)] from(3S,6R,S)-3-[(benzyloxycarbonyl)methyl]-6-methylmorpholine-2,5-dione.Macromol Chem Phys. 2002; 203:819-824.
[48] Liu Y,Yuan M,Deng X.Study on biodegradable polymers: synthesis and characterization of poly(DL-lactic acid-co-l-lysine) random copolymer.Eur Polym J.2003;39(5):977-983.
[49] Yakai F,Jens K,Doris K,et al.Enzyme-catalyzed ring-opening polymerization of 3(S)-isopro- Pylmorpholine-2,5-dione.Maeromol Rapid Comm. 1999;20(2):88-90.
[50] Yakai F,Doris K,Helmut K,et al.Lipase-catalyzed ring-opening polymerization of morholine-2,5-dione derivatives:Anovel route to the synthesis of poly(ester-amide)s.MacromoleeularChemistry and Physies.2000;201(18):2670-2675.
[51] Ouchi T,Shiratani M,Jinno M,et al.Synthesis of poly[(glycolic acid)-alt-(L-aspartic acid)] and its biodegradation behavior in vitro.Macromol Rapid Comm.1993;14(12):825-831.
[52] Feng Y,Klee D,Höcker H.Lipase catalyzed copolymerization of 3(S)-isopropylmorpholine- 2,5-dione and D,L-lactide.Macromol Biosci.2004; 4(6):587-590.
[53] Ouchi T,Hamada A,Ohya Y.Biodegradable microspheres having reactive groups prepared from L-lactic acid-depsipeptide copolymers.Chem Phys. 1999;200:436.
[54] [Hoffman JD,Miller RL.Kinetic of crystallization from the melt and chain folding in polyethylene fractions revisited: theory and experiment.Polymer. 1997;38(13): 3151-3212. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [3] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [4] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [5] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [6] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [7] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [8] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [9] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [10] | Xing Hao, Zhang Yonghong, Wang Dong. Advantages and disadvantages of repairing large-segment bone defect [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 426-430. |
| [11] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| [12] | Wang Hao, Chen Mingxue, Li Junkang, Luo Xujiang, Peng Liqing, Li Huo, Huang Bo, Tian Guangzhao, Liu Shuyun, Sui Xiang, Huang Jingxiang, Guo Quanyi, Lu Xiaobo. Decellularized porcine skin matrix for tissue-engineered meniscus scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3473-3478. |
| [13] | Mo Jianling, He Shaoru, Feng Bowen, Jian Minqiao, Zhang Xiaohui, Liu Caisheng, Liang Yijing, Liu Yumei, Chen Liang, Zhou Haiyu, Liu Yanhui. Forming prevascularized cell sheets and the expression of angiogenesis-related factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3479-3486. |
| [14] | Liu Chang, Li Datong, Liu Yuan, Kong Lingbo, Guo Rui, Yang Lixue, Hao Dingjun, He Baorong. Poor efficacy after vertebral augmentation surgery of acute symptomatic thoracolumbar osteoporotic compression fracture: relationship with bone cement, bone mineral density, and adjacent fractures [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3510-3516. |
| [15] | Liu Liyong, Zhou Lei. Research and development status and development trend of hydrogel in tissue engineering based on patent information [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3527-3533. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||