Chinese Journal of Tissue Engineering Research ›› 2016, Vol. 20 ›› Issue (27): 4090-4097.doi: 10.3969/j.issn.2095-4344.2016.27.020
Previous Articles Next Articles
Long-term aerobic exercise enhances the production of endogenous hydrogen sulfide in the kidney of spontaneously hypertensive rats
Wang Bing1, Hou Jun2, Gu Qi1
- 1School of Physical Education, Xi’an Technological University, Xi’an 710032, Shaanxi Province, China
2School of Physical Education, Luoyang Normal University, Luoyang 471022, Henan Province, China
-
Revised:2016-04-08Online:2016-06-30Published:2016-06-30 -
Contact:Gu Qi, Professor, School of Physical Education, Xi’an Technological University, Xi’an 710032, Shaanxi Province, China -
About author:Wang Bing, Master, Associate professor, School of Physical Education, Xi’an Technological University, Xi’an 710032, Shaanxi Province, China
CLC Number:
Cite this article
Wang Bing, Hou Jun, Gu Qi . Long-term aerobic exercise enhances the production of endogenous hydrogen sulfide in the kidney of spontaneously hypertensive rats[J]. Chinese Journal of Tissue Engineering Research, 2016, 20(27): 4090-4097.
share this article

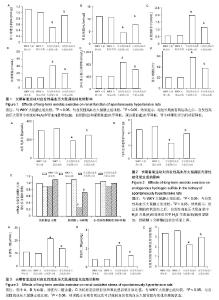
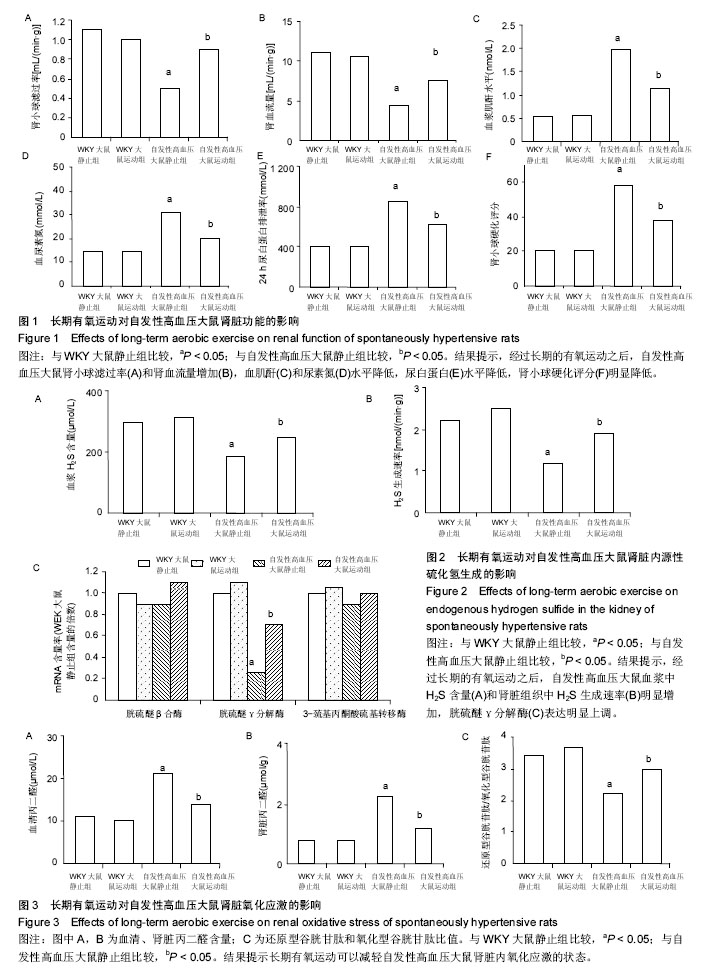
2.1 实验动物数量分析 实验分为4组,即WKY大鼠静止组、WKY大鼠运动组、自发性高血压大鼠静止组及自发性高血压大鼠运动组,每组12只。全部进入结果分析,无脱失。 2.2 长期有氧运动对自发性高血压大鼠血压和体质量的影响 如表1所示,与WKY大鼠静止组相比,自发性高血压大鼠的收缩压、舒张压、心率明显增高,体质量变化不大;而经过长期的有氧运动之后,自发性高血压大鼠的体质量、收缩压、舒张压明显降低,心率变化不明显。 WKY大鼠经过长期有氧运动后,体质量明显下降,但是收缩压、舒张压、心率变化不明显。 2.3 长期有氧运动改善自发性高血压大鼠的肾脏功能 与WKY大鼠静止组相比,自发性高血压大鼠肾小球滤过率(图1A)和肾血流量(图1B)降低,血肌酐(图1C)和尿素氮(图1D)水平增加,尿白蛋白(图1E)水平增加,肾小球硬化评分(图1F)明显增加;而经过长期的有氧运动之后,自发性高血压大鼠肾小球滤过率和肾血流量增加,血肌酐和尿素氮水平降低,尿白蛋白水平降低,肾小球硬化评分明显降低。 WKY大鼠经过长期有氧运动后,肾脏的各项指标没有明显变化。 2.4 长期有氧运动增加自发性高血压大鼠肾脏内源性H2S的生成 与WKY大鼠静止组相比,自发性高血压大鼠血浆中H2S含量明显减少(图2A);肾脏组织中H2S生成速率明显降低(图2B);肾脏中H2S合成相关酶(图2C):胱硫醚β合酶和3-巯基丙酮酸硫基转移酶的表达没有明显变化,而胱硫醚γ分解酶表达明显降低。而经过长期的有氧运动之后,自发性高血压大鼠血浆中H2S含量和肾脏组织中H2S生成速率明显增加;胱硫醚γ分解酶表达明显上调。 WKY大鼠经过长期有氧运动后,H2S生成的各项指标没有明显变化。 2.5 长期有氧运动减轻肾脏内氧化应激的状态 与WKY大鼠静止组相比,自发性高血压大鼠血清中(图3A)和肾脏(图3B)中丙二醛(脂质过氧化产物,氧化应激的标志之一)含量明显增加;还原型谷胱甘肽和氧化型谷胱甘肽比值明显降低(图3C)。而经过长期的有氧运动之后,自发性高血压大鼠血清中和肾脏中丙二醛含量明显降低;还原型谷胱甘肽和氧化型谷胱甘肽比值明显增加,提示长期有氧运动可以减轻自发性高血压大鼠肾脏内氧化应激的状态。 WKY大鼠经过长期有氧运动后,氧化应激的各项指标没有明显变化。"

| [1] Navaneethan SD, Schold JD, Arrigain S, et al. Body mass index and causes of death in chronic kidney disease. Kidney Int.2016;89:675-682. [2] Hall ME, do Carmo JM, da Silva AA, et al. Obesity, hypertension, and chronic kidney disease. Int J Nephrol Renovasc Dis.2014;7:75-88. [3] Townsend RR, Taler SJ. Management of hypertension in chronic kidney disease. Nat Rev Nephrol. 2015;11: 555-563. [4] Sabino JP, Traslaviña GA, Branco LG. Role of central hydrogen sulfide on ventilatory and cardiovascular responses to hypoxia in spontaneous hypertensive rats. Respir Physiol Neurobiol. 2016. pii: S1569-9048(16)30093-3. [5] Bidani AK, Polichnowski AJ, Loutzenhiser R, et al. Renal microvascular dysfunction, hypertension and CKD progression. Curr Opin Nephrol Hypertens. 2013; 22:1-9. [6] Hashimoto J, Ito S. Aortic Blood Flow Reversal Determines Renal Function: Potential Explanation for Renal Dysfunction Caused by Aortic Stiffening in Hypertension. Hypertension. 2015;66:61-67. [7] Lamina S, Okoye CG, Hanif SM. Randomised controlled trial: effects of aerobic exercise training programme on indices of adiposity and metabolic markers in hypertension. J Pak Med Assoc.2013;63:680-687. [8] Lee LL, Watson MC, Mulvaney CA, et al. The effect of walking intervention on blood pressure control: A systematic review. Int J Nurs Stud.2010;47:1545-1561. [9] Ghadieh AS, Saab B. Evidence for exercise training in the management of hypertension in adults. Can Fam Physician. 2015;61:233-239. [10] Sharman JE, La Gerche A, Coombes JS. Exercise and cardiovascular risk in patients with hypertension. Am J Hypertens. 2015;28:147-158. [11] Buys R, Avila A, Cornelissen VA. Exercise training improves physical fitness in patients with pulmonary arterial hypertension: a systematic review and meta-analysis of controlled trials. BMC Pulm Med. 2015; 15:40. [12] Ribeiro F, Costa R, Mesquita-Bastos J. Exercise training in the management of patients with resistant hypertension. World J Cardiol. 2015;7:47-51. [13] Chang Y, Cheng SY, Lin M, et al.The effectiveness of intradialytic leg ergometry exercise for improving sedentary life style and fatigue among patients with chronic kidney disease: a randomized clinical trial. Int J Nurs Stud.2010;47:1383-1388. [14] Henrique DM, Reboredo Mde M, Chaoubah A, et al. Aerobic exercise improves physical capacity in patients under chronic hemodialysis. Arq Bras Cardiol. 2010;94: 823-828. [15] Barcellos FC, Santos IS, Mielke GI, et al. Effects of exercise on kidney function among non-diabetic patients with hypertension and renal disease: randomized controlled trial. BMC Nephrol. 2012;13:90. [16] Maia RC, Sousa LE, Santos RA, et al. Time-course effects of aerobic exercise training on cardiovascular and renal parameters in 2K1C renovascular hypertensive rats. Braz J Med Biol Res. 2015;48:1010-1022. [17] MacKinnon HJ, Feehally J, Smith AC. A review of the role of exercise and factors affecting its uptake for people with chronic kidney disease (CKD) not requiring renal replacement therapy. Prilozi. 2015;36:37-46. [18] Aucella F, Valente GL, Catizone L. The role of physical activity in the CKD setting. Kidney Blood Press Res. 2014;39:97-106. [19] Gu Q, Zhao L, Ma YP, et al. Contribution of mitochondrial function to exercise-induced attenuation of renal dysfunction in spontaneously hypertensive rats. Mol Cell Biochem. 2015;406:217-225. [20] Be?towski J. Hydrogen sulfide in pharmacology and medicine--An update. Pharmacol Rep. 2015;67: 647-658. [21] Snijder PM, Frenay AR, Koning AM, et al. Sodium thiosulfate attenuates angiotensin II-induced hypertension, proteinuria and renal damage. Nitric Oxide. 2014;42:87-98. [22] Huang P, Chen S, Wang Y, et al. Down-regulated CBS/H2S pathway is involved in high-salt-induced hypertension in Dahl rats. Nitric Oxide. 2015;46: 192-203. [23] Holwerda KM, Burke SD, Faas MM, et al. Hydrogen sulfide attenuates sFlt1-induced hypertension and renal damage by upregulating vascular endothelial growth factor. J Am Soc Nephrol. 2014;25:717-725. [24] Ahmad FU, Sattar MA, Rathore HA, et al. Hydrogen sulphide and tempol treatments improve the blood pressure and renal excretory responses in spontaneously hypertensive rats. Ren Fail. 2014; 36:598-605. [25] Xin HG, Zhang BB, Wu ZQ, et al. Consumption of hydrogen-rich water alleviates renal injury in spontaneous hypertensive rats. Mol Cell Biochem. 2014;392:117-124. [26] Xia M, Chen L, Muh RW, et al. Production and actions of hydrogen sulfide, a novel gaseous bioactive substance, in the kidneys. J Pharmacol Exp Ther. 2009;329:1056-1062. [27] Yang G, Wu L, Jiang B, et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322 (5901):587-590. [28] Heyman SN, Brezis M, Greenfeld Z, et al. Protective role of furosemide and saline in radiocontrast-induced acute renal failure in the rat. Am J Kidney Dis .1989;14: 377-385. [29] Tanaka T, Nangaku M. The role of hypoxia, increased oxygen consumption, and hypoxia-inducible factor-1 alpha in progression of chronic kidney disease. Curr Opin Nephrol Hypertens. 2010;19:43-50. [30] Muzaffar S, Shukla N, Bond M, et al. Exogenous hydrogen sulfide inhibits superoxide formation, NOX-1 expression and Rac1 activity in human vascular smooth muscle cells. J Vasc Res .2008;45:521-528. [31] Xie ZZ, Liu Y, Bian JS. Hydrogen Sulfide and Cellular Redox Homeostasis.Oxid Med Cell Longev. 2016: 6043038. [32] Sen U, Munjal C, Qipshidze N, et al. Hydrogen sulfide regulates homocysteine-mediated glomerulosclerosis. Am J Nephrol. 2010;31:442-455. [33] Lo Faro ML, Fox B, Whatmore JL, et al. Hydrogen sulfide and nitric oxide interactions in inflammation. Nitric Oxide. 2014;41:38-47. [34] Manning RD Jr, Tian N, Meng S. Oxidative stress and antioxidant treatment in hypertension and the associated renal damage. Am J Nephrol. 2005;25: 311-317. [35] Yoon JW, Pahl MV, Vaziri ND. Spontaneous leukocyte activation and oxygen-free radical generation in end-stage renal disease. Kidney Int. 2007;71:167-172. [36] Pan X, Zhang Y, Tao S. Effects of Tai Chi exercise on blood pressure and plasma levels of nitric oxide, carbon monoxide and hydrogen sulfide in real-world patients with essential hypertension. Clin Exp Hypertens. 2015;37:8-14. [37] Gu Q, Wang B, Zhang XF, et al. Contribution of hydrogen sulfide and nitric oxide to exercise-induced attenuation of aortic remodeling and improvement of endothelial function in spontaneously hypertensive rats. Mol Cell Biochem. 2013;375:199-206. [38] 姚璐,张缨,张连峰.跑台运动在动物实验研究中的应用[J].中国比较医学杂志,2010,20(6):75-81. [39] Gu Q, Wang B, Zhang XF, et al. Contribution of renin-angiotensin system to exercise-induced attenuation of aortic remodeling and improvement of endothelial function in spontaneously hypertensive rats. Cardiovasc Pathol. 2014;23:298-305. [40] Gu Q, Wang B, Zhang XF, et al. Chronic aerobic exercise training attenuates aortic stiffening and endothelial dysfunction through preserving aortic mitochondrial function in aged rats. Exp Gerontol. 2014;56:37-44. [41] Yu H, Xu H, Liu X, et al. Superoxide Mediates Depressive Effects Induced by Hydrogen Sulfide in Rostral Ventrolateral Medulla of Spontaneously Hypertensive Rats. Oxid Med Cell Longev. 2015; 2015:927686. [42] Sun Y, Huang Y, Zhang R, et al. Hydrogen sulfide upregulates KATP channel expression in vascular smooth muscle cells ofspontaneously hypertensive rats. J Mol Med (Berl). 2015;93(4):439-455. [43] Sun L, Jin H, Sun L, et al. Hydrogen sulfide alleviates myocardial collagen remodeling in association with inhibition of TGF-β/Smad signaling pathway in spontaneously hypertensive rats. Mol Med. 2015;20: 503-515. [44] Sikora M, Drapala A, Ufnal M. Exogenous hydrogen sulfide causes different hemodynamic effects in normotensive andhypertensive rats via neurogenic mechanisms. Pharmacol Rep. 2014;66(5):751-758. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [3] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [4] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [5] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [6] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [7] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [8] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [9] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [10] | Xing Hao, Zhang Yonghong, Wang Dong. Advantages and disadvantages of repairing large-segment bone defect [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 426-430. |
| [11] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| [12] | Wang Hao, Chen Mingxue, Li Junkang, Luo Xujiang, Peng Liqing, Li Huo, Huang Bo, Tian Guangzhao, Liu Shuyun, Sui Xiang, Huang Jingxiang, Guo Quanyi, Lu Xiaobo. Decellularized porcine skin matrix for tissue-engineered meniscus scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3473-3478. |
| [13] | Mo Jianling, He Shaoru, Feng Bowen, Jian Minqiao, Zhang Xiaohui, Liu Caisheng, Liang Yijing, Liu Yumei, Chen Liang, Zhou Haiyu, Liu Yanhui. Forming prevascularized cell sheets and the expression of angiogenesis-related factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3479-3486. |
| [14] | Liu Chang, Li Datong, Liu Yuan, Kong Lingbo, Guo Rui, Yang Lixue, Hao Dingjun, He Baorong. Poor efficacy after vertebral augmentation surgery of acute symptomatic thoracolumbar osteoporotic compression fracture: relationship with bone cement, bone mineral density, and adjacent fractures [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3510-3516. |
| [15] | Liu Liyong, Zhou Lei. Research and development status and development trend of hydrogel in tissue engineering based on patent information [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3527-3533. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||