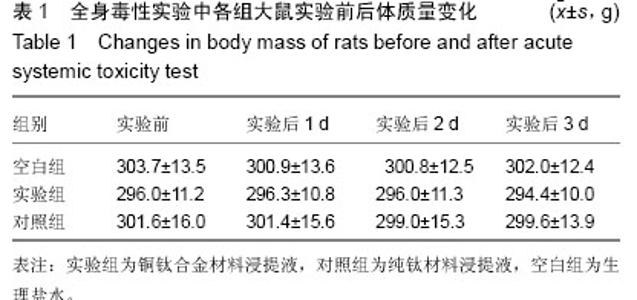
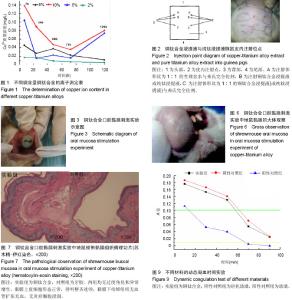
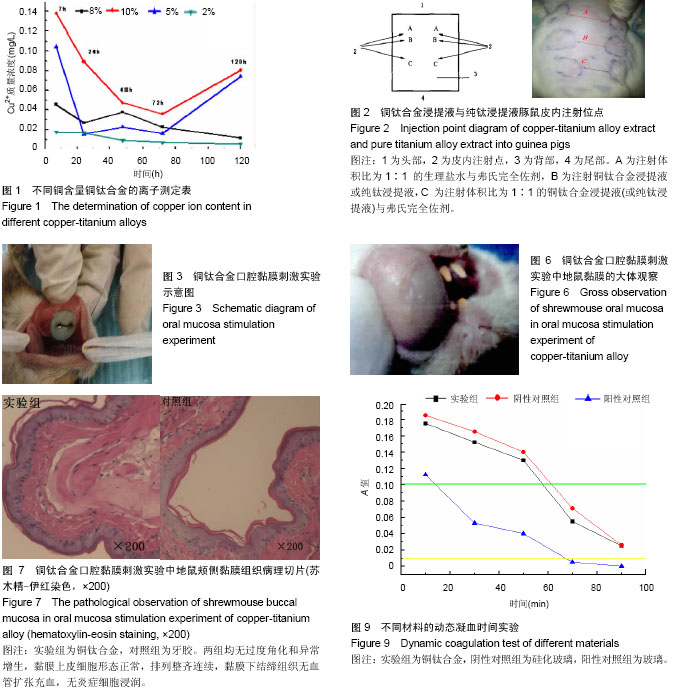
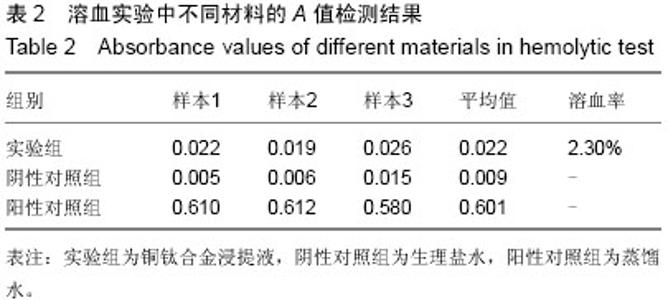
| [1] 冯颖芳,康浩芳,张震.钛合金医用植入物材料的研究及应用[J].稀有金属,2001,25(5): 349-354.
[2] Costerton JW,Stewart PS,Greenberg EP.Bacterial biofilms: a common cause of persistent infections.Science.1999; 284(5418):1318-1322.
[3] Liu XY,Chu PK,Ding CX.Surface modification of titanium, titanium alloys, and related materials for biomedical applications.Mat Sci Eng R.2004;47:49-121.
[4] De Bastiani G,Aldegheri R,Renzi Brivio L.The treatment of fractures with a dynamic axial fixator.J Bone Joint Surg Br.1984;66(4):538-545.
[5] Zhang E,Li F,Wang H,et al.A new antibacterial titanium-copper sintered alloy: preparation and antibacterial property.Mater Sci Eng C Mater Biol Appl. 2013;33(7): 4280-4287.
[6] Liu J,Li F,Liu C,et al.Effect of Cu content on the antibacterial activity of titanium-copper sintered alloys.Mater Sci Eng C Mater Biol Appl.2014;35:392-400.
[7] Liu J,Zhang X,Wang H,et al.The antibacterial properties and biocompatibility of a Ti-Cu sintered alloy for biomedical application.Biomed Mater.2014;9(2):025013.
[8] 8 Petite H,Viateau V,Bensaid W,et al.Tissue-engineered boneregeneration.Nat Biotechnol.2000;18(9):959-963.
[9] Niinomi M.Recent metallic materials for biomedical applications.Met Mater Trans.2002;33:477-486.
[10] 上海生物材料研究测试中心译国际标准化组织7406技术报告:牙科材料的生物学评价[J].口腔材料器械杂志,1995,13(4):194.
[11] 杨晓芳,奚廷斐.生物材料生物相容性评价进展[J].生物医学工程学杂志,2001,18(1):123-128.
[12] 陈治清.口腔材料学[M].北京:人民卫生出版社,2003:1-3.
[13] 郝和平.医疗器械生物学评价标准实施指南[M].北京:标准出版社,2000:114.
[14] 李佐臣,李常亮,裘松波,等.外科植入TAMZ合金生物学性能评价[J].稀有金属材料与工程,1998,27(1):59-61.
[15] Chiba A,Kumagai K,Takeda H,et al.Mechanical Properties of Forged Low-Niand C-Containing Co-Cr-Mo BiomedicalImplant Al1oy.Mater Sci Forum.2005;475-479: 2317-2322.
[16] ISO10993-1 Biological evaluation of medical devices-part1: Evaluation and Testing[S].3rd ed. Switzerland:ISO.
[17] 李晓征,白玉盛.骨髓细胞形态学临床带教的体会与思考[J].临床和实验医学杂志,2007,6(7):179-181.
[18] 余贯华,计剑,王东安,等.两种新型聚氨酯涂层材料的血液相容性研究[J].生物医学工程学杂志,2004,21(2):184. |