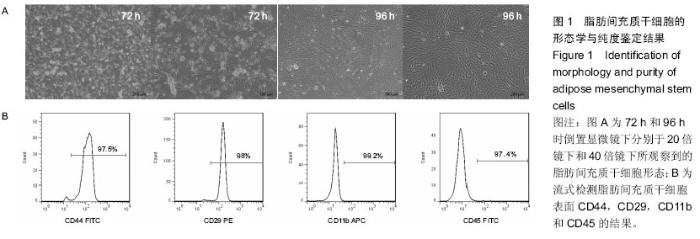
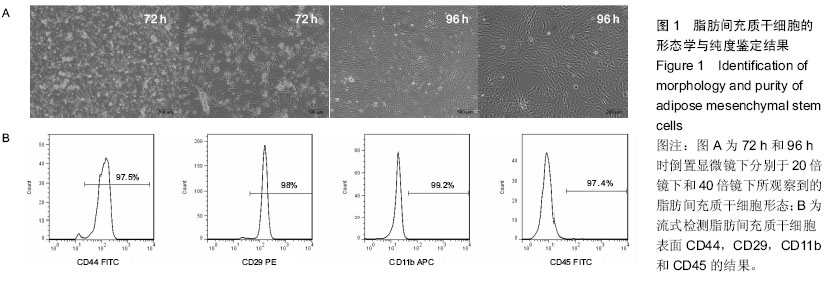
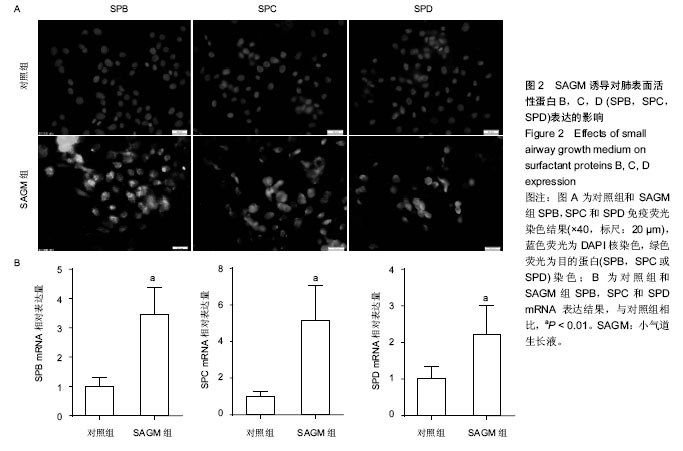
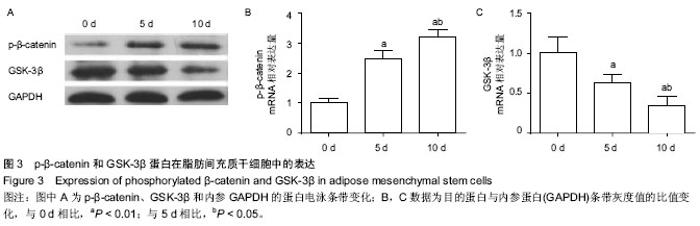
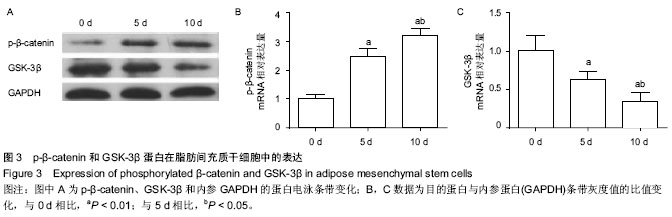
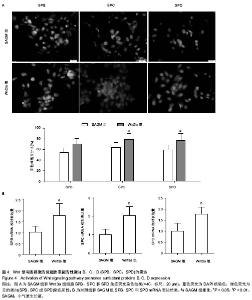
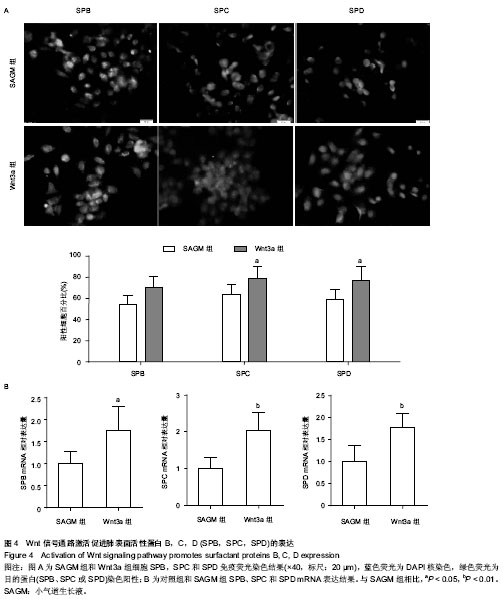
| [1] Guillot L, Nathan N, Tabary O, et al. Alveolar epithelial cells: master regulators of lung homeostasis. Int J Biochem Cell Biol. 2013;45(11):2568-2573.
[2] Rozycki HJ. Potential contribution of type I alveolar epithelial cells to chronic neonatal lung disease. Front Pediatr 2014;2: 45.
[3] Hoffman AM, Ingenito EP. Alveolar epithelial stem and progenitor cells: emerging evidence for their role in lung regeneration. Curr Med Chem. 2012;19(35):6003-6008.
[4] Dietl P, Haller T, Frick M. Spatio-temporal aspects, pathways and actions of Ca(2+) in surfactant secreting pulmonary alveolar type II pneumocytes. Cell Calcium 2012;52(3-4):296-302.
[5] Minteer DM, Marra KG, Rubin JP. Adipose stem cells: biology, safety, regulation, and regenerative potential. Clin Plastic Surg. 2015;42(2):169-179.
[6] Tsuji W, Rubin JP, Marra KG. Adipose-derived stem cells: Implications in tissue regeneration. World J Stem Cells. 2014;26;6(3):312-321.
[7] Lim MH, Ong WK, Sugii S. The current landscape of adipose-derived stem cells in clinical applications. Expert Rev Mol Med. 2014;16:e8.
[8] Forcales SV. Potential of adipose-derived stem cells in muscular regenerative therapies. Front Aging Neurosci. 2015; 7:123.
[9] Perdisa F, Gostynska N, Roffi A, Filardo G, Marcacci M, Kon E. Adipose-derived mesenchymal stem cells for the treatment of articular cartilage: a systematic review on preclinical and clinical evidence. Stem Cells Int. 2015;2015:597652.
[10] Kim N, Cho SG. New strategies for overcoming limitations of mesenchymal stem cell-based immune modulation. Int J Stem Cells. 2015;8(1):54-68.
[11] Escacena N, Quesada-Hernandez E, Capilla-Gonzalez V, et al. Bottlenecks in the efficient use of advanced therapy medicinal products based on mesenchymal stromal cells. Stem Cells Int. 2015;2015:895714.
[12] Yi H, Kang KY, Kim Y, et al. Human adipose-derived mesenchymal stem cells attenuate collagen antibody-induced autoimmune arthritis by inducing expression of FCGIIB receptors. BMC Musculoskelet Disord. 2015;16:170.
[13] Gonzalez-Fernandez ML, Perez-Castrillo S, Ordas-Fernandez P, et al. Study on viability and chondrogenic differentiation of cryopreserved adipose tissue-derived mesenchymal stromal cells for future use in regenerative medicine. Cryobiology. 2015.
[14] Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5(5):362-369.
[15] Han S, Sun HM, Hwang KC, et al. Adipose-derived stromal vascular fraction cells: update on clinical utility and efficacy. Crit Rev Eukaryot Gene Expr. 2015;25(2):145-152.
[16] Lv FJ, Tuan RS, Cheung KM, et al. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32(6):1408-1419.
[17] Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317.
[18] Ma N, Gai H, Mei J, et al. Bone marrow mesenchymal stem cells can differentiate into type II alveolar epithelial cells in vitro. Cell Boil Int. 2011;35(12):1261-1266.
[19] Shi C, Lv T, Xiang Z, et al. Role of Wnt/beta-catenin signaling in epithelial differentiation of lung resident mesenchymal stem cells. J Cell Biochem. 2015;116(8):1532-1539.
[20] Zhou Q, Ye X, Sun R, Matsumoto Y, et al. Differentiation of mouse induced pluripotent stem cells into alveolar epithelial cells in vitro for use in vivo. Stem Cells Transl Med. 2014;3(6): 675-85.
[21] Collu GM, Hidalgo-Sastre A, Brennan K. Wnt-Notch signalling crosstalk in development and disease. Cell Mol Life Sci. 2014; 71(18):3553-3567.
[22] Pataki CA, Couchman JR, Brabek J. Wnt signaling cascades and the roles of syndecan proteoglycans. J Histochem Cytochem. 2015;63(7):465-80.
[23] Kang KS, Robling AG. New Insights into Wnt-Lrp5/6-beta- Catenin Signaling in Mechanotransduction. Front Endocrinol. 2014;5:246.
[24] Ring A, Kim YM, Kahn M. Wnt/catenin signaling in adult stem cell physiology and disease. Stem Cell Rev. 2014;10(4):512- 525.
[25] Serman L, Nikuseva Martic T, Serman A, et al. Epigenetic alterations of the Wnt signaling pathway in cancer: a mini review. Bosn J Basic Med Sci. 2014;14(4):191-194.
[26] Monga SP. Role and regulation of beta-catenin signaling during physiological liver growth. Gene Expr. 2014;16(2): 51-62.
[27] Liu AR, Liu L, Chen S, et al. Activation of canonical wnt pathway promotes differentiation of mouse bone marrow-derived MSCs into type II alveolar epithelial cells, confers resistance to oxidative stress, and promotes their migration to injured lung tissue in vitro. J Cell Physiol. 2013; 228(6):1270-1283.
[28] Liu A, Chen S, Cai S, et al. Wnt5a through noncanonical Wnt/JNK or Wnt/PKC signaling contributes to the differentiation of mesenchymal stem cells into type II alveolar epithelial cells in vitro. PLoS One. 2014;9(3):e90229.
[29] Wang C, Zhu H, Sun Z, et al. Inhibition of Wnt/beta-catenin signaling promotes epithelial differentiation of mesenchymal stem cells and repairs bleomycin-induced lung injury. Am J Physiol Cell Physiol 2014; 307(3):C234-C244. |