Chinese Journal of Tissue Engineering Research ›› 2013, Vol. 17 ›› Issue (41): 7335-7340.doi: 10.3969/j.issn.2095-4344.2013.41.023
Tissue-engineered nerve for repair of peripheral nerve injuries
Fu Chong-yang1, Zhao Jia2, Qu Wei1
- 1Department of Hand Surgery and Microsurgery, First Affiliated Hospital of Dalian Medical University, Dalian 116011, Liaoning Province, China; 2ICU, Sixth People’s Hospital of Dalian, Dalian 116031, Liaoning Province, China
-
Received:2013-07-05Revised:2013-08-23Online:2013-10-08Published:2013-11-01 -
About author:Fu Chong-yang☆, M.D., Associate chief physician, Associate professor, Department of Hand Surgery and Microsurgery, First Affiliated Hospital of Dalian Medical University, Dalian 116011, Liaoning Province, China fuchongyang@hotmail.com
CLC Number:
Cite this article
Fu Chong-yang, Zhao Jia, Qu Wei. Tissue-engineered nerve for repair of peripheral nerve injuries[J]. Chinese Journal of Tissue Engineering Research, 2013, 17(41): 7335-7340.
share this article
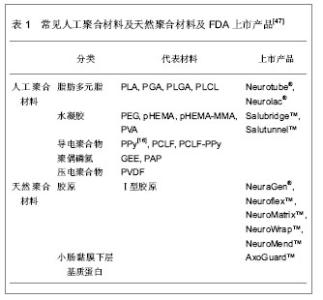
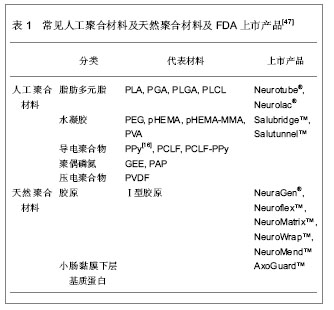
2.1 种子细胞 由于种子细胞具有促进和引导轴突再生的功能,因此对种子细胞的深入研究成为组织工程化周围神经是否能够成功的关键一环。 许旺细胞:许旺细胞作为神经干内主要的非神经元活性细胞,是损伤神经远端惟一分裂、增殖并对轴突再生有重要作用的细胞,具有神经营养、趋化和使神经再生纤维成熟的重要功能,是周围神经组织工程的核心。许旺细胞的分离、培养、纯化、传代均已取得可喜的成就,Gansmuller等[3]已培养出MSC 80永生化许旺细胞系。目前,种植有许旺细胞的人工神经修复周围神经缺损已在多个实验室取得成功[4-6],因此,许旺细胞作为种子细胞在人工神经中的核心地位已形成共识[7]。但自体外周许旺细胞为终末期细胞,存在增殖能力差,体外分离、培养困难和活性下降,来源缺乏,需追加一次手术取材等缺点,限制了临床的推广使用[8];而异体许旺细胞移植会被受体组织强烈排斥[9-10],在体内难以存活。如何从个体化治疗向规模化治疗迈进并实现组织工程技术的产业化,这就为种子细胞研究提出了新的挑战。 骨髓基质干细胞:骨髓基质干细胞是具有多向分化潜能的组织工程前体细胞。骨髓基质干细胞已经证实可以分化为汗腺[11]、心肌[12]、上皮[13]、胶质细胞及神经元细胞[14-16]。近年来,也有将骨髓基质干细胞在体外、体内诱导分化为许旺样细胞的报道。Tohill等[17]最先成功将骨髓基质干细胞培养分化为许旺细胞放入导管移植修复神经缺损。随后,一系列研究均报道将骨髓基质干细胞分化为许旺细胞作为种子细胞植入,取得成效[18-22]。但对于骨髓基质干细胞分化的许旺细胞,仍然有许多亟待突破的禁区:首先,人们对其分化及调控的分子机制知之甚少;其次,骨髓基质干细胞体外转化为许旺细胞属于将中胚层细胞系转化为外胚层细胞系,难度大,比率低,并且存在逆向转化现象,要满足临床治疗,还需要建立规范的分离纯化和扩增技术;第三,体外处理的骨髓基质干细胞需要连续给予各种生物因子,尚存在性状不稳定,长期存活困难的问题。因此,就目前的研究现状来看,组织工程化神经的种子细胞仍然存在许多难题,故目前还没有一种人工神经能应用于临床[23]。 脂肪源性干细胞:脂肪源性干细胞最先从大鼠脂肪组织基质血管中发现[24],而后从人吸脂的废弃脂肪组织中分离得到[25]。脂肪源性干细胞表现出多向分化潜能,可以分化为软骨、骨、脂肪和肌肉组织[26-27]。脂肪源性干细胞也起源于中胚层,90%细胞表面标记物与骨髓基质干细胞相似[27-28],但它在体内含量大,更易分离培养[29-30]。在特定的细胞培养基中,他们可以分化成许旺样细胞,这些优势使得脂肪源性干细胞更适合应用于神经组织工程。Kingham等[31]最先应用神经胶质细胞生长因子2、碱性成纤维细胞生长因子、血小板源性生长因子和forskolin共同作用,发现培养的脂肪源性干细胞变为纺锤型态,外形与许旺细胞类似。通过测定,发现这些细胞表达GFAP,S100及p75等神经胶质细胞特征标记物,这提示,脂肪源性干细胞可以体外诱导分化为许旺样细胞。而后,许多研究也表明,脂肪源性干细胞转化的许旺细胞可以表达神经源性生长因子,生成有髓纤维,并且在周围神经损伤的模型中,促进了轴突再生[32-34]。与骨髓基质干细胞相同,从脂肪源性干细胞转化为许旺细胞的应用与组织工程的实用性也存在争论。首先性状不稳定,转化细胞移植于体内存活时间不定。其次转化部分需要大量序贯的生物因子,并非生理性的许旺细胞替代。因此维持脂肪源性干细胞的稳定性及长期体内追踪是将来研究的方向。应用脂肪源性干细胞作为种子细胞构建周围神经尚需要大量的实验来验证。 神经干细胞:神经干细胞具有分化为神经元与神经胶质细胞的潜能,故其也被应用于周围神经再生动物模型[35]。Murakami 等[36]将神经干细胞放置于硅胶管内修复神经缺损,发现可以促进神经轴突再生。Heine等[37]在慢性神经横断模型中,用神经干细胞移植修复同样促进神经再生。但是也有研究证明体外将未分化神经干细胞注入导管后移植修复神经离断伤,并未取得预期的结果[38-39]。同时,未分化神经干细胞移植引起的神经肿瘤也应引起重视[40],临床上移植异源性神经干细胞导致肿瘤形成也有报道[41]。 胚胎干细胞:胚胎干细胞是一种高度未分化的全能细胞,具有极强的增殖与分化能力,加入诱导因子后能分化为体内任何一种细胞。胚胎干细胞作为种子细胞可以大量制备,满足组织工程需要[42-43]。由于胚胎干细胞强大的分化能力,在体内过度增殖,将它在体外预处理成神经祖细胞再将其移植至体内修复大鼠坐骨神经缺损,明显促进神经再生,经细胞标记物鉴定,其成功分化成许旺细胞[44-45]。但目前有研究证实即使将很少量的幼稚胚胎干细胞移植至体内,即可形成畸胎瘤[46]。且人胚胎干细胞来源存在伦理学争议,同时异种胚胎干细胞移植还需克服免疫排斥等问题,需要应用免疫抑制剂。 2.2 生物材料 模拟周围神经的自然结构,将种子细胞与生物材料构建成类似Bungner带的结构,并起到细胞外基质替代物的作用,是周围神经组织工程的核心内容。作为支架材料必须具有良好的生物相容性、表面活性;有利于形成良好的血运;一定强度的三维空间结构,避免瘢痕组织侵扰;一定大小的孔径和孔隙率;促进轴突再生的生物学活性。 人工聚合材料:人工聚合材料在生产过程中通过共聚、交联技术改变相对分子质量及分子成分,可以改变其降解时间及特性,故它可以广泛地应用于神经组织工程。人工聚合材料包括脂肪多元脂类、水凝胶类、导电聚合物类、聚偶磷氮类及压电聚合物类,见表1。 天然聚合材料:天然聚合材料包括胶原、小肠黏膜下层基质蛋白、丝纤蛋白、琼脂糖、藻酸盐、壳聚糖、纤维连接蛋白及纤维蛋白。与人工聚合材料相比,天然材料具有更好的生物相容性,故通常通过交联技术与人工聚合材料结合,或者作为中空神经导管的填充物使用,见表1。"

导电性纳米碳管:导电性纳米碳管具有典型的层状中空结构,是由管状纳米级石墨构成的生物材料。根据圈曲石墨的层数分为单壁导电性纳米碳管(single-walled CNT, SWNTs)与多壁导电性纳米碳管(Multi-walled CNT, MWNTs)[48]。 导电性纳米碳管与人工聚合物相交连,在体外可以明显促进神经轴突的再生[49-50]。导电性纳米碳管具有如下特点:①体积非常小,能够更好地与细胞接触。②具有良好的弹性与强度,能在组织修复过程中具有组织的柔韧性,又保持支架结构的完整。③具有良好的导电性能,且可调控。④具有稳定的光谱性状,可以被追踪、监测,了解治疗情况。⑤具有携带各种生物活性物质的能力[51-52],故将导电性纳米碳管应用于周围神经组织工程具有广阔的前景,将可能实现工程化组织产品临床应用的重大突破。 2.3 构建周围神经组织的技术 基因修饰技术:周围神经损伤后,神经再生需要各种生物生长因子的参与。种子细胞于体外进行基因转染后再将其移植于体内,使其释放需要的各种神经营养因子,促进神经再生[53-55]。由于神经损伤后,神经离断两端会表达各种生长因子,并且远端的组织表达水平要高于近端,形成近低远高的浓度梯度[56]。这种短暂的浓度梯度是体内神经再生的信号机制。 尽管应用基因修饰技术已经在周围神经损伤的模型上取得了成功的疗效,但是基因修饰细胞释放的神经营养因子提高了其体内浓度,破坏了原有的浓度梯度变化,反而会产生“陷阱效应”干扰神经纤维再生,甚至抑制神经再生[57-60]。为此,基因修饰技术应加以调控,使其在局部释放生理需要剂量浓度的生长因子,保持体内应有的信号梯度,这是学者努力的方向。 静电纺丝技术:静电纺丝技术是生产直径在微米到纳米范围的连续纤维的方法,可以通过控制聚合物溶液浓度、黏度、配比等参数改变纤维的尺寸大小;另外,改变电场的强度和方式可以使所产生的支架结构与形态不同。由于静电纺丝支架具有更类似于天然的细胞外基质的结构,其高生物相容性、降解性及极高的表面积/容积比率,为细胞的黏附、迁移、增殖和分化功能提供更有利的环境[61],近年来备受关注。但是目前的研究仍仅限于种子细胞和支架生物相容性等的研究,将其用于临床修复神经损伤,还有很多问题需要解决。 促进神经再生方法:构建的组织工程周围神经最终要移植于体内,成为神经的一部分。尽管目前的显微外科技术及设备已经相对成熟,但是神经功能完全恢复正常的实验研究尚未见报道。周围神经损伤后,相应节段的中枢神经元凋亡、移植神经生长缓慢、靶器官机能丧失等这些都是影响神经功能恢复的因素。目前,大量的研究都关注于如何加速神经轴突的再生,如应用各种外源性生长因子、对神经施加光、电刺激等[62-63]。对于如何保护神经元,防止靶器官失用,也应是神经组织功能研究的一部分,成为将来研究的热点问题。"

| [1] Lundborg G. A 25-year perspective of peripheral nerve surgery: evolving neuroscientific concepts and clinical significance. J Hand Surg Am. 2000;25(3):391-414.
[2] Evans PJ, Midha R, Mackinnon SE. The peripheral nerve allograft: a comprehensive review of regeneration and neuroimmunology. Prog Neurobiol. 1994;43(3): 187-233.
[3] Gansmuller A, Clerin E, Krüger F,et al. Tracing transplanted oligodendrocytes during migration and maturation in the shiverer mouse brain. Glia. 1991;4(6):580-590.
[4] Ansselin AD, Fink T, Davey DF. Peripheral nerve regeneration through nerve guides seeded with adult Schwann cells. Neuropathol Appl Neurobiol. 1997 ;23(5):387-398.
[5] Keeley R, Atagi T, Sabelman E,et al. Synthetic nerve graft containing collagen and synthetic Schwann cells inproves functional, electrophysiological, and histological parameters of peripheral nerve regeneration. Restor Neurol Neurosci. 1993;5(5):353-366.
[6] Kim DH, Connolly SE, Kline DG,et al. Labeled Schwann cell transplants versus sural nerve grafts in nerve repair. J Neurosurg. 1994;80(2):254-260.
[7] Hudson TW, Evans GR, Schmidt CE. Engineering strategies for peripheral nerve repair. Orthop Clin North Am. 2000;31(3):485-498.
[8] Ringe J, Kaps C, Burmester GR,et al. Stem cells for regenerative medicine: advances in the engineering of tissues and organs. Naturwissenschaften. 2002;89(8):338-351.
[9] Rodríguez FJ, Verdú E, Ceballos D,et al. Nerve guides seeded with autologous schwann cells improve nerve regeneration. Exp Neurol. 2000;161(2):571-584.
[10] Spierings E, Vleggeert-Lankamp CL, Marani E,et al. Allorecognition of artificial nerve guides filed with human Schwann cells: an in vitro piloot study. Transplantation. 2000;69(3):455-456.
[11] Sheng Z, Fu X, Cai S,et al. Regeneration of functional sweat gland-like structures by transplanted differentiated bone marrow mesenchymal stem cells. Wound Repair Regen. 2009;17(3):427-435.
[12] Orlic D, Kajstura J, Chimenti S,et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410(6829):701-705.
[13] Oswald J, Boxberger S, Jørgensen B,et al. Mesenchymal stem cells can be differentiated into endothelial cells in vitro.Stem Cells. 2004;22(3):377-384.
[14] Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999;96(19):10711-10716.
[15] Hofstetter CP, Schwarz EJ, Hess D,et al. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci U S A. 2002;99(4):2199-2204.
[16] Jiang Y, Jahagirdar BN, Reinhardt RL,et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418(6893):41-49.
[17] Tohill M, Mantovani C, Wiberg M,et al. Rat bone marrow mesenchymal stem cells express glial markers and stimulate nerve regeneration. Neurosci Lett. 2004;362(3):200-203.
[18] Choi BH, Zhu SJ, Kim BY,et al. Transplantation of cultured bone marrow stromal cells to improve peripheral nerve regeneration. Int J Oral Maxillofac Surg. 2005;34(5):537-542.
[19] Caddick J, Kingham PJ, Gardiner NJ,et al. Phenotypic and functional characteristics of mesenchymal stem cells differentiated along a Schwann cell lineage.Glia. 2006;54(8):840-849.
[20] Keilhoff G, Goihl A, Stang F,et al. Peripheral nerve tissue engineering: autologous Schwann cells vs. transdifferentiated mesenchymal stem cells.Tissue Eng. 2006 ;12(6):1451-1465.
[21] Mahay D, Terenghi G, Shawcross SG. Schwann cell mediated trophic effects by differentiated mesenchymal stem cells. Exp Cell Res. 2008;314(14):2692-2701.
[22] Brohlin M, Mahay D, Novikov LN,et al. Characterisation of human mesenchymal stem cells following differentiation into Schwann cell-like cells. Neurosci Res. 2009;64(1):41-49.
[23] Evans GR. Challenges to nerve regeneration. Semin Surg Oncol. 2000;19(3):312-318.
[24] Hollenberg CH, Vost A. Regulation of DNA synthesis in fat cells and stromal elements from rat adipose tissue. J Clin Invest. 1969;47(11):2485-2498.
[25] Van RL, Bayliss CE, Roncari DA. Cytological and enzymological characterization of adult human adipocyte precursors in culture. J Clin Invest. 1976;58(3):699-704.
[26] Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5(5):362-369.
[27] Strem BM, Hicok KC, Zhu M,et al. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54(3):132-141.
[28] De Ugarte DA, Morizono K, Elbarbary A,et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174(3):101-109.
[29] Pittenger MF, Mackay AM, Beck SC,et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143-147.
[30] Aust L, Devlin B, Foster SJ,et al. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy. 2004;6(1):7-14.
[31] Kingham PJ, Kalbermatten DF, Mahay D,et al. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp Neurol. 2007;207(2):267-274.
[32] Xu Y, Liu L, Li Y,et al. Myelin-forming ability of Schwann cell-like cells induced from rat adipose-derived stem cells in vitro. Brain Res. 2008;1239:49-55.
[33] Chi GF, Kim MR, Kim DW,et al. Schwann cells differentiated from spheroid-forming cells of rat subcutaneous fat tissue myelinate axons in the spinal cord injury. Exp Neurol. 2010;222(2):304-317.
[34] di Summa PG, Kalbermatten DF, Pralong E,et al. Long-term in vivo regeneration of peripheral nerves through bioengineered nerve grafts. Neuroscience. 2011;181:278-291.
[35] Bithell A, Williams BP. Neural stem cells and cell replacement therapy: making the right cells. Clin Sci (Lond). 2005;108(1):13-22.
[36] Murakami T, Fujimoto Y, Yasunaga Y,et al. Transplanted neuronal progenitor cells in a peripheral nerve gap promote nerve repair. Brain Res. 2003;974(1-2):17-24.
[37] Heine W, Conant K, Griffin JW,et al. Transplanted neural stem cells promote axonal regeneration through chronically denervated peripheral nerves. Exp Neurol. 2004;189(2):231-240.
[38] Luís AL, Rodrigues JM, Geuna S,et al. Use of PLGA 90:10 scaffolds enriched with in vitro-differentiated neural cells for repairing rat sciatic nerve defects.Tissue Eng Part A. 2008;14(6):979-993.
[39] Amado S, Rodrigues JM, Luís AL,et al. Effects of collagen membranes enriched with in vitro-differentiated N1E-115 cells on rat sciatic nerve regeneration after end-to-end repair. J Neuroeng Rehabil. 2010;7:7.
[40] Radtke C, Redeker J, Jokuszies A,et al. In vivo transformation of neural stem cells following transplantation in the injured nervous system. J Reconstr Microsurg. 2010;26(3):211-212.
[41] Amariglio N, Hirshberg A, Scheithauer BW,et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 2009;6(2):e1000029.
[42] Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292(5819):154-156.
[43] Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78(12): 7634-7638.
[44] Cui L, Jiang J, Wei L,et al. Transplantation of embryonic stem cells improves nerve repair and functional recovery after severe sciatic nerve axotomy in rats. Stem Cells. 2008;26(5):1356-1365.
[45] Liu Q, Spusta SC, Mi R,et al. Human neural crest stem cells derived from human ESCs and induced pluripotent stem cells: induction, maintenance, and differentiation into functional schwann cells. Stem Cells Transl Med. 2012;1(4):266-278.
[46] Nussbaum J, Minami E, Laflamme MA,et al. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. FASEB J. 2007;21(7):1345-1357.
[47] Nguyen TY, Liu H. A Review of Current Advances in Biomaterials for Neural Tissue Regeneration. Recent Patents on Biomedical Engineering.2013;6(1):29-39.
[48] Tran PA, Zhang L, Webster TJ. Carbon nanofibers and carbon nanotubes in regenerative medicine. Adv Drug Deliv Rev. 2009;61(12):1097-1114.
[49] Malarkey EB, Fisher KA, Bekyarova E,et al. Conductive single-walled carbon nanotube substrates modulate neuronal growth. Nano Lett. 2009;9(1):264-268.
[50] Jin GZ, Kim M, Shin US,et al. Neurite outgrowth of dorsal root ganglia neurons is enhanced on aligned nanofibrous biopolymer scaffold with carbon nanotube coating. Neurosci Lett. 2011;501(1):10-14.
[51] Olakowska E, Woszczycka-Korczyńska I, J?drzejowska-Szypu?ka H,et al. Application of nanotubes and nanofibres in nerve repair. A review. Folia Neuropathol. 2010;48(4):231-237.
[52] 向宁,王光林.以导电性纳米碳管为支架的组织工程神经研究进展[J]. 中国修复重建外科杂志, 2011,25(11):1389-1392.
[53] Wong LF, Goodhead L, Prat C,et al. Lentivirus-mediated gene transfer to the central nervous system: therapeutic and research applications. Hum Gene Ther. 2006;17(1):1-9.
[54] Hendriks WT, Eggers R, Carlstedt TP,et al. Lentiviral vector-mediated reporter gene expression in avulsed spinal ventral root is short-term, but is prolonged using an immune "stealth" transgene. Restor Neurol Neurosci. 2007;25(5-6):585-599.
[55] Tannemaat MR, Eggers R, Hendriks WT,et al. Differential effects of lentiviral vector-mediated overexpression of nerve growth factor and glial cell line-derived neurotrophic factor on regenerating sensory and motor axons in the transected peripheral nerve. Eur J Neurosci. 2008;28(8):1467-1479.
[56] Trupp M, Rydén M, Jörnvall H,et al. Peripheral expression and biological activities of GDNF, a new neurotrophic factor for avian and mammalian peripheral neurons. J Cell Biol. 1995;130(1):137-148.
[57] Santosa KB, Jesuraj NJ, Viader A,et al. Nerve allografts supplemented with schwann cells overexpressing glial-cell-line-derived neurotrophic factor. Muscle Nerve. 2013;47(2):213-223.
[58] Tannemaat MR, Eggers R, Hendriks WT,et al. Differential effects of lentiviral vector-mediated overexpression of nerve growth factor and glial cell line-derived neurotrophic factor on regenerating sensory and motor axons in the transected peripheral nerve. Eur J Neurosci. 2008;28(8):1467-1479.
[59] Blits B, Carlstedt TP, Ruitenberg MJ,et al. Rescue and sprouting of motoneurons following ventral root avulsion and reimplantation combined with intraspinal adeno-associated viral vector-mediated expression of glial cell line-derived neurotrophic factor or brain-derived neurotrophic factor. Exp Neurol. 2004;189(2):303-316.
[60] Shakhbazau A, Kawasoe J, Hoyng SA,et al. Early regenerative effects of NGF-transduced Schwann cells in peripheral nerve repair. Mol Cell Neurosci. 2012;50(1):103-112.
[61] 张伟,李明,傅强. 静电纺丝纤维支架在神经组织工程中的应用进展[J]. 中华外科杂志,2010,48(20):1584-1587.
[62] Rochkind S, Geuna S, Shainberg A. Chapter 25: Phototherapy in peripheral nerve injury: effects on muscle preservation and nerve regeneration. Int Rev Neurobiol. 2009;87:445-464.
[63] Panetsos F, Avendaño C, Negredo P,et al. Neural prostheses: electrophysiological and histological evaluation of central nervous system alterations due to long-term implants of sieve electrodes to peripheral nerves in cats. IEEE Trans Neural Syst Rehabil Eng. 2008;16(3):223-232. |
| [1] | Wang Jing, Xiong Shan, Cao Jin, Feng Linwei, Wang Xin. Role and mechanism of interleukin-3 in bone metabolism [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1260-1265. |
| [2] | Xiao Hao, Liu Jing, Zhou Jun. Research progress of pulsed electromagnetic field in the treatment of postmenopausal osteoporosis [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(8): 1266-1271. |
| [3] | Tian Chuan, Zhu Xiangqing, Yang Zailing, Yan Donghai, Li Ye, Wang Yanying, Yang Yukun, He Jie, Lü Guanke, Cai Xuemin, Shu Liping, He Zhixu, Pan Xinghua. Bone marrow mesenchymal stem cells regulate ovarian aging in macaques [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 985-991. |
| [4] | Hui Xiaoshan, Bai Jing, Zhou Siyuan, Wang Jie, Zhang Jinsheng, He Qingyong, Meng Peipei. Theoretical mechanism of traditional Chinese medicine theory on stem cell induced differentiation [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1125-1129. |
| [5] | An Weizheng, He Xiao, Ren Shuai, Liu Jianyu. Potential of muscle-derived stem cells in peripheral nerve regeneration [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1130-1136. |
| [6] | Fan Yiming, Liu Fangyu, Zhang Hongyu, Li Shuai, Wang Yansong. Serial questions about endogenous neural stem cell response in the ependymal zone after spinal cord injury [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1137-1142. |
| [7] | Hou Jingying, Guo Tianzhu, Yu Menglei, Long Huibao, Wu Hao. Hypoxia preconditioning targets and downregulates miR-195 and promotes bone marrow mesenchymal stem cell survival and pro-angiogenic potential by activating MALAT1 [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1005-1011. |
| [8] | Zhou Ying, Zhang Huan, Liao Song, Hu Fanqi, Yi Jing, Liu Yubin, Jin Jide. Immunomodulatory effects of deferoxamine and interferon gamma on human dental pulp stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1012-1019. |
| [9] | Liang Xuezhen, Yang Xi, Li Jiacheng, Luo Di, Xu Bo, Li Gang. Bushen Huoxue capsule regulates osteogenic and adipogenic differentiation of rat bone marrow mesenchymal stem cells via Hedgehog signaling pathway [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1020-1026. |
| [10] | Wang Jifang, Bao Zhen, Qiao Yahong. miR-206 regulates EVI1 gene expression and cell biological behavior in stem cells of small cell lung cancer [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1027-1031. |
| [11] | Liu Feng, Peng Yuhuan, Luo Liangping, Wu Benqing. Plant-derived basic fibroblast growth factor maintains the growth and differentiation of human embryonic stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1032-1037. |
| [12] | Wen Dandan, Li Qiang, Shen Caiqi, Ji Zhe, Jin Peisheng. Nocardia rubra cell wall skeleton for extemal use improves the viability of adipogenic mesenchymal stem cells and promotes diabetes wound repair [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1038-1044. |
| [13] | Zhu Bingbing, Deng Jianghua, Chen Jingjing, Mu Xiaoling. Interleukin-8 receptor enhances the migration and adhesion of umbilical cord mesenchymal stem cells to injured endothelium [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1045-1050. |
| [14] | Luo Xiaoling, Zhang Li, Yang Maohua, Xu Jie, Xu Xiaomei. Effect of naringenin on osteogenic differentiation of human periodontal ligament stem cells [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1051-1056. |
| [15] | Wang Xinmin, Liu Fei, Xu Jie, Bai Yuxi, Lü Jian. Core decompression combined with dental pulp stem cells in the treatment of steroid-associated femoral head necrosis in rabbits [J]. Chinese Journal of Tissue Engineering Research, 2022, 26(7): 1074-1079. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||