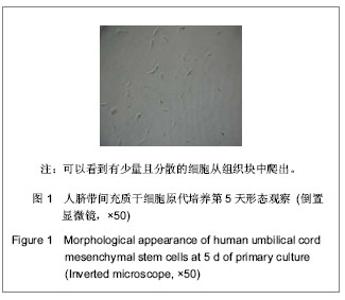
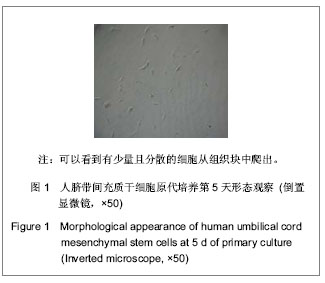
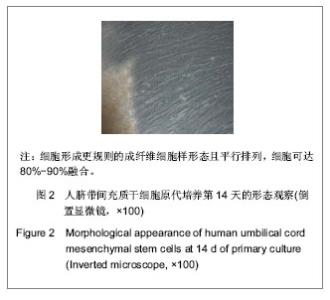
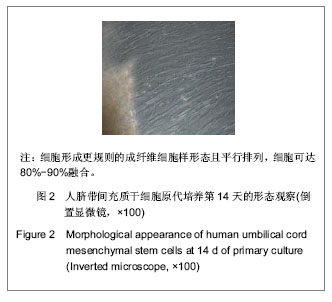
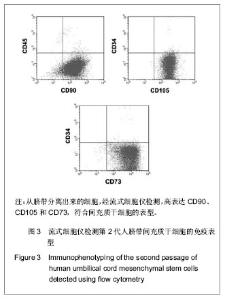
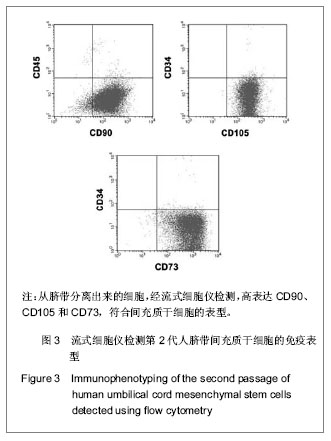
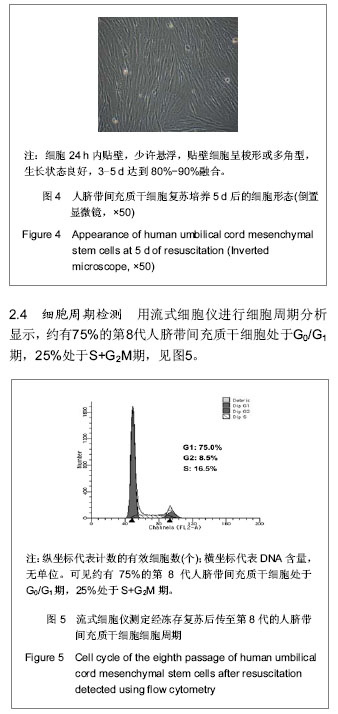
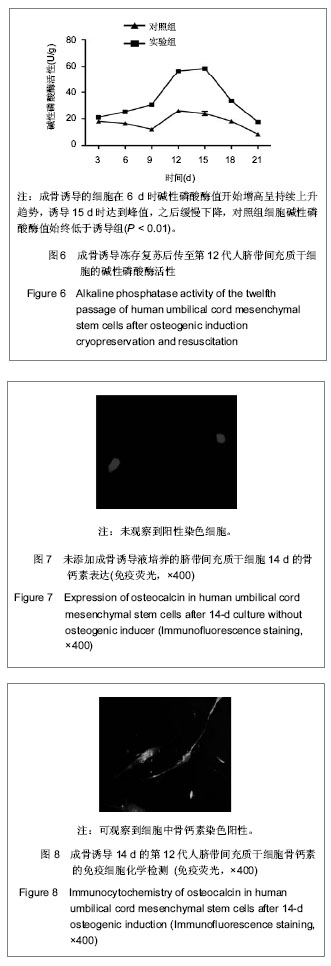
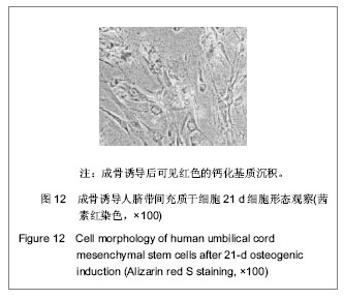
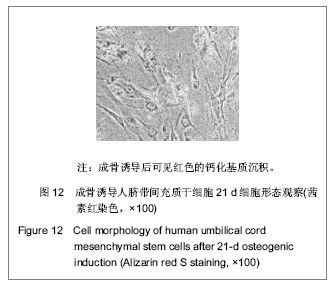
| [1] Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143-147.
[2] Li ZY, Chen L, Liu L, et al. Odontogenic potential of bone marrow mesenchymal stem cells. J Oral Maxillofac Surg. 2007;65(3):494-500.
[3] Secco M, Zucconi E, Vieira NM, et al. Multipotent stem cells from umbilical cord: cord is richer than blood! Stem Cells.2008; 26(1):146-150.
[4] Wang L, Tran I, Seshareddy K, et al. A comparison of human bone marrow-derived mesenchymal stem cells and human umbilical cord-derived mesenchymal stromal cells for cartilage tissue engineering. Tissue Eng Part A. 2009;15(8):2259-2266.
[5] 吕江涛,田少奇,孙康,等.血小板裂解液对人脐带间充质干细胞体外成骨分化的影响[J].中国组织工程研究,2012,16(41): 7637-7641.
[6] 张镇,田少奇,张才龙,等.携带人骨形态发生蛋白2可诱导慢病毒载体转染人脐带间充质干细胞的成骨[J].中国组织工程研究, 2012,16(14):2545-2549.
[7] 曲志国,野向阳,林辉,等.人脐带间充质干细胞诱导成骨及治疗骨缺损[J].中国组织工程研究与临床康复,2011,15(45):8503- 8507.
[8] Kadner A, SP Hoerstrup, J Tracy, et al. Human umbilical cord cells: a new cell source for cardiovascular tissue engineering. Ann Thorac Surg.2002;74:S1422–S1428.
[9] Wang L, Ott L, Seshareddy K, et al. Musculoskeletal tissue engineering with human umbilical cord mesenchymal stromal cells. Regen Med. 2011;6(1):95-109.
[10] Can A, Karahuseyinoglu S. Concise review: human umbilical cord stroma with regard to the source of fetus-derived stem cells. Stem Cells. 2007;25(11):2886-2895.
[11] Peng J, Wang Y, Zhang L, et al. Human umbilical cord Wharton's jelly- derived mesenchymal stem cells differentiate into a Schwann-cell phenotype and promote neurite outgrowth in vitro. Brain Res Bull. 2011;84(3):235-243.
[12] 刘忠,李素萍,杨宏友,等.人脐带间充质干细胞体外分化成骨细胞的研究[J].中国输血杂志, 2011,24(5):376-382.
[13] 扎拉嘎胡,陈莉,兰晓霞,等.脐带间充质干细胞向成骨细胞分化的潜能[J].中国组织工程研究与临床康复,2010,14(40): 7439-7442.
[14] 何绍清,罗振宇,刘秋英,等.人脐带间充质干细胞分离培养及向脂肪与成骨细胞的分化[J].中国组织工程研究与临床康复,2010, 14(14):2492-2496.
[15] 孙国栋,李志忠,王晶,等.人脐带间充质干细胞的分离培养及向成骨成脂分化的实验研究[J]. 西安交通大学学报:医学版,2010, 14(2):143-147.
[16] 赵磊,王文加,付常皓,等.人脐带间充质干细胞的分离培养及成脂成骨分化[J].中国老年学杂志,2010,29(17):2460-2462.
[17] 野向阳,李相军,徐岩,等.人脐带间充质干细胞体外成骨及其免疫学特征[J]. 国组织工程研究与临床康复,2009,13(36): 7029- 7033.
[18] Hsieh JY, Fu YS, Chang SJ, et al. Functional module analysis reveals differential osteogenic and stemness potentials in human mesenchymal stem cells from bone marrow and Wharton's jelly of umbilical cord. Stem Cells Dev. 2010;19(12): 1895-1910.
[19] Han Y, Chai J, Sun T, et al. Differentiation of human umbilical cord mesenchymal stem cells into dermal fibroblasts in vitro. Biochem Biophys Res Commun. 2011;413(4):561-565.
[20] Karahuseyinoglu S, Cinar O, Kilic E, et al. Biology of stem cells in human umbilical cord stroma: in situ and in vitro Surveys. Stem Cells. 2007;25(2):319-331.
[21] De Bruyn C, Najar M, Raicevic G, et al. A rapid, simple, and reproducible method for the isolation of mesenchymal stromal cells from Wharton’s jelly without enzymatic treatment. Stem Cells. 2011;20(3):547-557.
[22] Peng J, Wang Y, Zhang L, et al. Human umbilical cord Wharton's jelly- derived mesenchymal stem cells differentiate into a Schwann-cell phenotype and promote neurite outgrowth in vitro. Brain Res Bull. 2011;84(3):235-243.
[23] Tong CK, Vellasamy S, Tan BC, et al. Generation of mesenchymal stem cell from human umbilical cord tissue using combination of enzymatic and mechanical disassociation method. Cell Biol Int. 2011;35(3):221-226.
[24] Chen MY, Lie PC, Li ZL, et al. Endothelial differentiation of Wharton's jelly-derived mesenchymal stem cells in comparison with bone marrow-derived mesenchymal stem cells. Exp Hematol. 2009;37(5):629-640.
[25] Wang HS, Hung SC, Peng ST, et al. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells. 2004;22(7):1330-1337.
[26] Yu J, Deng Z, Shi J, et al. Differentiation of Dental Pulp Stem Cells into Regular-Shaped Dentin-Pulp Complex Induced by Tooth Germ Cell Conditioned Medium. Tissue Eng. 2006; 12(11):3097-3105.
[27] Huang CH, Tseng WY, Yao CC, et al. Glucosamine promotes osteogenic differentiation of dental pulp stem cells through modulating the level of the transforming growth factor-beta type I receptor. J Cell Physiol. 2010;225(1):140-151.
[28] Lindroos B, Mäenpää K, Ylikomi T, et al. Characterisation of human dental stem cells and buccal mucosa fibroblasts. Biochem Biophys Res Commun. 2008;368(2):329-335.
[29] Phuc PV, Nhung TH, Loan DT, et al. Differentiating of banked human umbilical cord blood-derived mesenchymal stem cells into insulin-secreting cells. In Vitro Cell Dev Biol Anim. 2011; 47(1):54-63. |