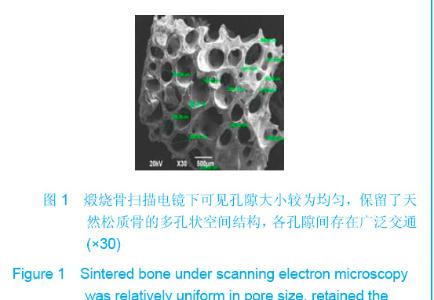
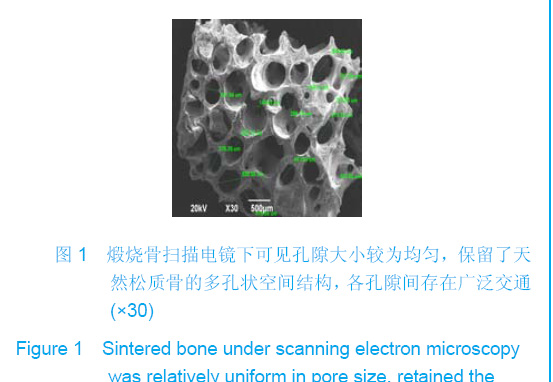
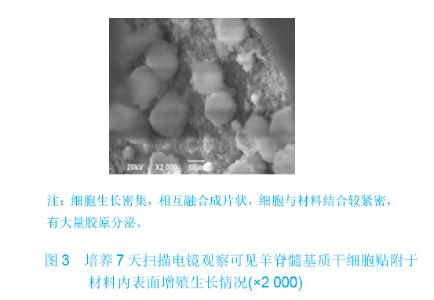
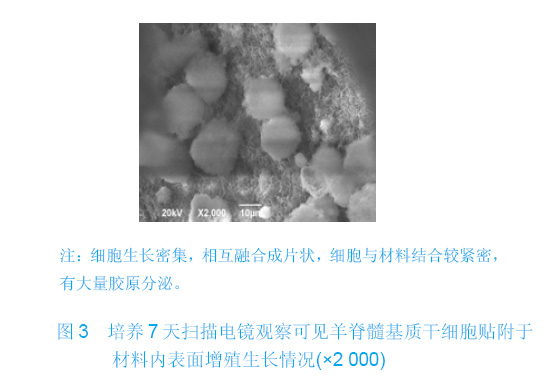
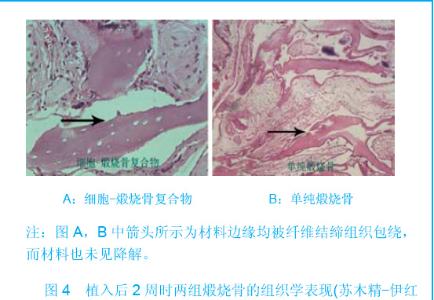
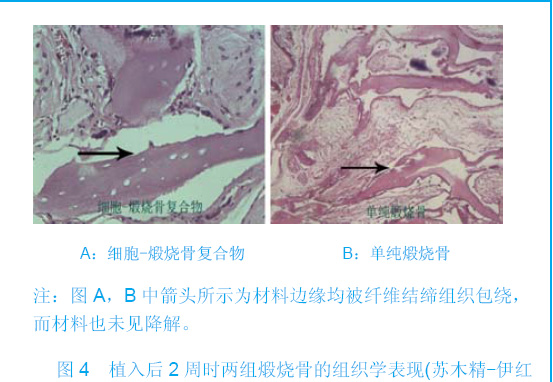
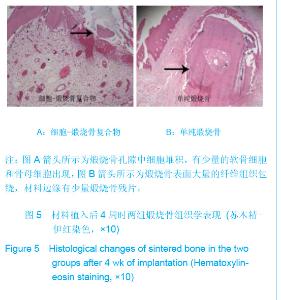
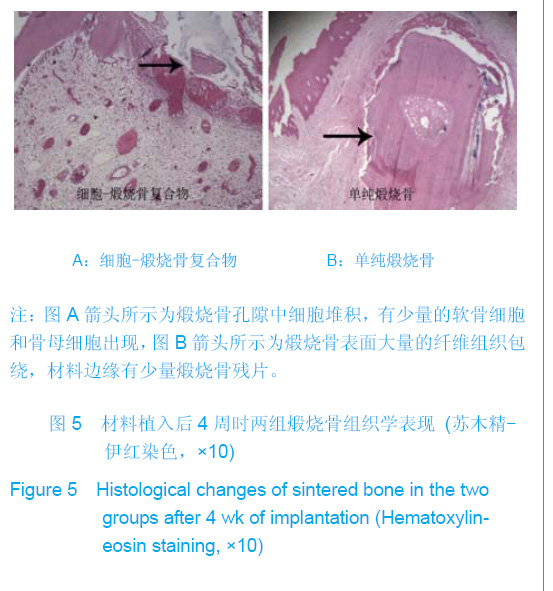
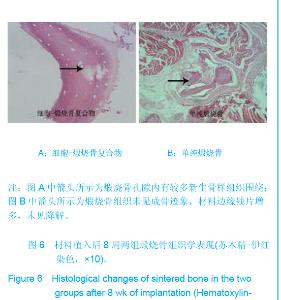
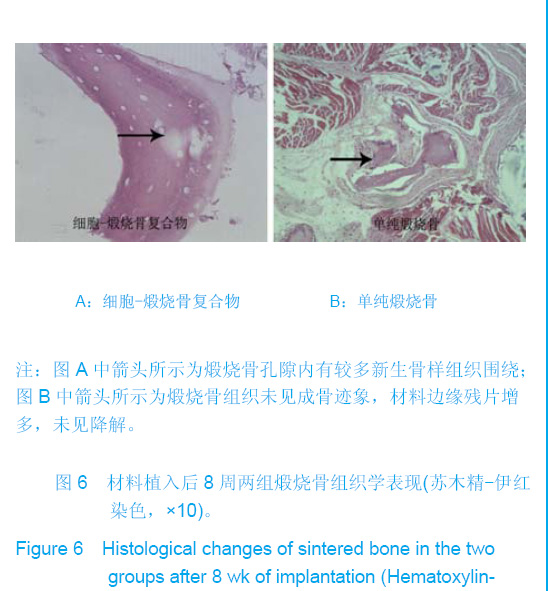
| [1]Zhang X, Yao Y, Liu YF. Linchuang Kouqiang Yixue Zazhi. 2010; 26(5):292-294.张淅,姚遥,刘迎飞.牙周炎致骨缺损的植骨治疗[J].临床口腔医学杂志,2010,26(5):292-294.[2]Tian ZF, Qin SJ, Wang RF, et al. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2008;12(15):2801-2805.田志逢,秦书俭,王瑞芳,等.骨髓间充质干细胞复合异种骨基质明胶修复大鼠桡骨缺损[J].中国组织工程研究与临床康复,2008, 12(15):2801-2805.[3]Feng QL, Cui FZ, Zhang W. Zhongguo Yixue Kexueyuan Xuebao.2002;24(2):124-128.冯庆玲,崔福斋,张伟.纳米羟基磷灰石/胶原骨修复材料[J].中国医学科学院报, 2002,24(2):124-128.[4]Yang CB, He HY. Zhongguo Shiyong Kouqiangke Zazhi. 2012;3(5):182-185.杨川博,何惠宇.牙槽骨缺损修复治疗新进展[J].中国实用口腔科杂志,2012,3(5):182-185.[5]Li G, Gao CY, Jin LJ. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2009;13(12):2217-2221.李罡,高春阳,金莲锦.异种脱蛋白松质骨支架与经诱导骨髓间充质干细胞的生物相容性[J].中国组织工程研究与临床康复, 2009,13(12):2217-2221.[6]Lazarus HM, Koc ON, Devine SM, et al. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marro Transplant. 2005;11(5):389-398.[7]Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005; 105(4):1815-1822.[8]Xu HF, He HY, Tang XX, et al. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2012;16(6):958-962.许慧芬,何惠宇,唐小雪,等.物理联合化学或化学方法处理去抗原异种松质骨支架与骨髓间充质干细胞的细胞相容性[J].中国组织工程研究与临复,2012,16(6):958-962.[9]The Ministry of Science and Technology of the People’s Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30.[10]Cui J, He HY. Urumqi: Xinjiang Yike Daxue. 2011.崔杰,何惠宇.去抗原异种松质骨支架材料的制备及性能研究[D].乌鲁木齐:新疆医科大学,2011.[11]Erdemli O, Captug O, Bilgili H, et al. In vitro and in vivo evaluation of the effects of demineralized bone matrix or calcium sulfate additionto polycaprolactone-bioglass composites. J Mater Sci Mater Med. 2010;21(1):295-308.[12]Jhn K, Braunstein V, Furlong PI, et al. A rapid method for the generation of uniform acellular bone explants. J Orthop Surg Res. 2010;10(5):28-32.[13]Tang XX, He HY, Xu HF. Effects of two separation methods on biological characteristics of sheep bone marrow mesenchymal stem cells. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2011;15(49):9137-9140.[14]Liu BY, Ma XH, Li NY. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2008;12(41):8055-8058.刘斌钰,马晓红,李宁毅,等.煅烧骨的生物相容性及细胞相容性评价[J].中国组织工程研究与临床康复,2008,12(41):8055-8058.[15]Mastrogiacom OM, Muraglia A, Komlev V, et al. Tissue engineering of bone: search for a better scaffold. Orthod Craniofac Res. 2005;8(4):277-284.[16]Erdemli O, Captug O, Bilgili H, et al. In vitro and in vivo evaluation of the effects of demineralized bone matrix or calcium sulfate additionto polycaprolactone-bioglass composites. J Mater Sci Mater Med. 2010;21(1): 295-308.[17]Jähn K, Braunstein V, Furlong PI, et al. A rapid method for the generation of uniform acellular bone explants. J Orthop Surg Res. 2010;10(5):28-32.[18]Chen LQ, Li NY, Liu BY, et al. Shanghai Kouqiang Yixue. 2007; 16(3):255-258.陈立强,李宁毅,刘斌钰,等.犬脱钙骨基质复合骨髓间充质干细胞的体外培养[J].上海口腔医学,2007,16(3):255-258[19]Li NY, Chen LQ, Chen T, et al. Huaxi Kouqiang Yixue Zazhi. 2007;25(4):408-411.李宁毅,陈立强,陈涛,等.富血小板血浆与带血管肌筋膜在组织工程骨血管化中的作用[J].华西口腔医学杂志, 2007,25(4): 408-411.[20]Wilson CE, Kruyt MC, de Bruijn JD, et al. A new in vivo screening model for posterior spinal bone formation: comparison of ten calcium phosphate ceramic material treatments. Biomaterials. 2006;27(3):302-314.[21]Miks AG, Temenoff JS. Formation of highly porous biodegradable scaffolds for tissue engineering. Electron J Biotechnol. 2000;3(2):114-119.[22]Xu YH, Shi XQ, Hu YY. Disi Junyi Daxue Xuebao. 2002; 23(3): 223-226.许永华,施新猷,胡蕴玉,同种异体成骨细胞-煅烧骨复合物动物体内植入[J].第四军医大学学报,2002,23(3):223-226.[23]Zhou X, Zuo ZH, Cao YL. Zhonghua Shoushuxue Zazhi. 2006; 10(4):316-332.周晓,左朝晖,曹谊林.组织工程骨修复骨缺损的研究进展[J].中华手术学杂志,2006,10(4):316-332.[24]Kruyt M, De Bruijn J, Rouwkema J, et al. Analysis of the dynamics of bone formation, effect of cell seeding density, and potential of allogeneic ceils in cell-based bone tissue engineering in goats. Tissue Eng Part A. 2008;14(6): 1081-1088.[25]van Gaalen SM, de Bruijn JD, Wilson CE, et al. Relating cell proliferation to in vivo bone formation in porous Ca/P scaffolds. J Biomed Mater Res A. 2010;92A(1):303.[26]Han XS, Tan YJ, Huang YL. Shanghai Kouqiang Yixue. 2010;12(19):641-646.韩雪松,谭鸾君,黄远亮,等.Beagle犬骨髓基质细胞复合A-PCPC裸鼠皮下成骨的实验研究[J].上海口腔医学,2010,12(19): 641-646.[27]Yuan J,Wang M. Zhongguo Kouqiang Hemian Waike Zazhi. 2005;3(3):207-211.袁捷,殷德民,王敏,等.犬骨髓基质干细胞复合珊瑚羟基磷灰石皮下成骨的实验研究[J].中国口腔颌面外科杂志, 2005,3(3): 207-211.[28]Liu XM,Tian ZF,Zhang XZ. Zhongguo Zuzhi Gongcheng Yanjiu. 2012;16(25):4566-4570.刘喜民,田志逢,张小玲,等.骨基质明胶/煅烧骨基质重组人工骨对骨髓间充质干细胞成骨潜能的影响[J].中国组织工程研究, 2012,16(25):4566-4570.[29]Canter HI, Vargel I, Mavili ME. Reconstruction of mandibular defects using autografts combined with demineralized bone matrix and cancellous allograft. J Craniofac Surg. 2007;18(1): 95-100. |