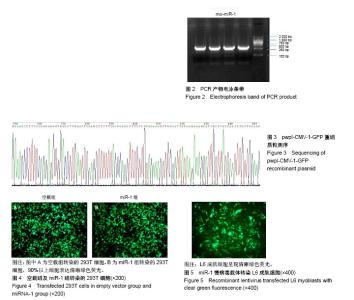
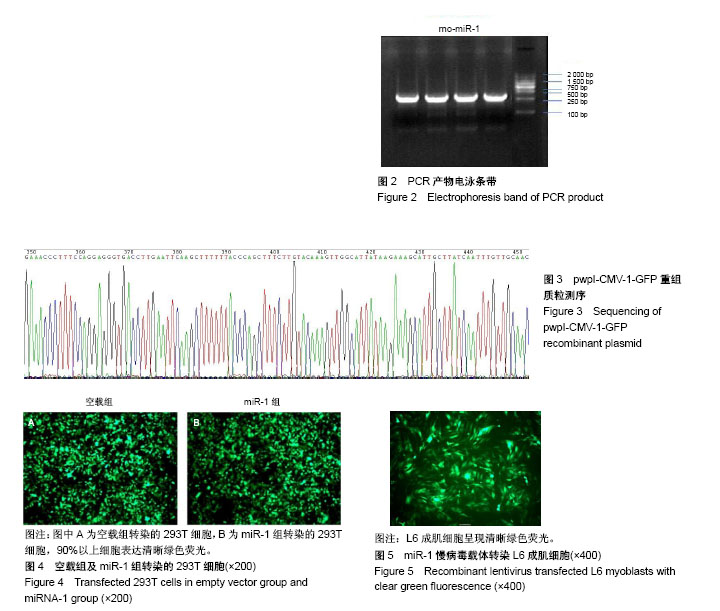
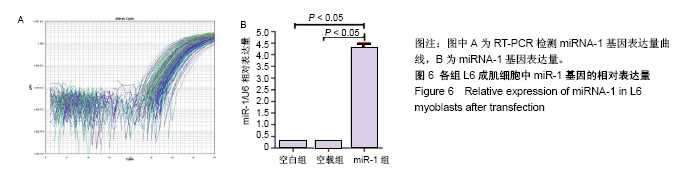
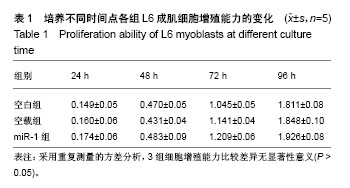
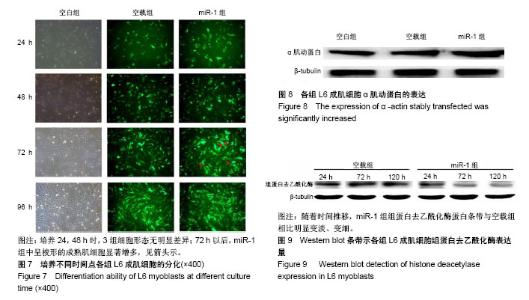
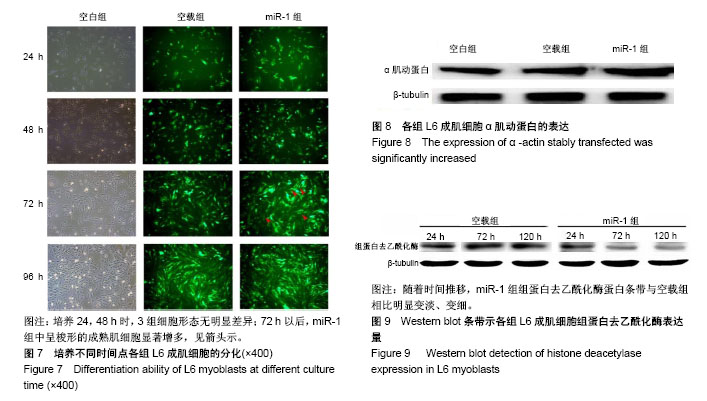
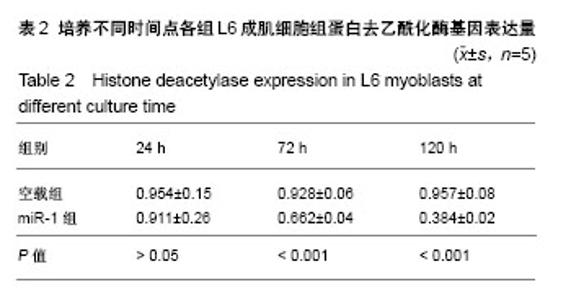
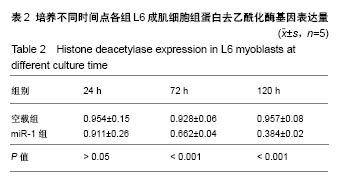
| [1] 徐建广,顾玉东,李继峰,等.大鼠失神经支配骨骼肌及其运动终板退变观察[J].中华显微外科杂志,1999,22(3):215. [2] 路来金,卢志远,刘志敏,等.手虎口挛缩征的诊断和治疗[J].中华显微外科杂志,1998,21(4):295-296.[3] Ambros V.The functions of animal microRNAs.Nature. 2004;431:350-355.[4] Bartel DP.MicroRNAs: genomics, biogenesis, mechanism, and function. Cell.2004;116(2):281-297.[5] Eisenberg I,Eran A,Nishino I,et al.Distinctive patterns of microRNA expression in primary muscular disorders.Proc Natl Acad Sci U S A. 2007;104(43):17016-17021.[6] Fritegotto C,Ferrati C,Pegoraro V,et al.Micro-RNA expression in muscle and fiber morphometry in myotonic dystrophy type 1. Neurol Sci. 2017; 38(4):619-625.[7] Fujii R,Osaka E,Sato K,et al.MiR-1 Suppresses Proliferation of Osteosarcoma Cells by Up-regulating p21 PAX3. Cancer Genomics Proteomics.2019;16(1):71-79. [8] Townley-Tilson WH,Callis TE,Wang D.MicroRNAs 1, 133, and 206: critical factors of skeletal and cardiac muscle development, function,and disease.Int J Biochem Cell Biol. 2010;42(8):1252-1255.[9] Luo S,Chen Y,He R,et al. Rescuing infusion of miRNA-1 prevents cardiac remodeling in a heart-selective miRNA deficient mouse.Biochem Biophys Res Commun. 2018;495(1):607-613.[10] Xue X,Shi X,Dong H,et al. Delivery of microRNA-1 inhibitor by dendrimer-based nanovector: An early targeting therapy for myocardial infarction in mice.Nanomedicine. 2018;14(2):619-631. [11] Midrio M.The denervated muscle: facts and hypotheses. A historical review.Eur J Appl Physiol. 2006;98(1):1-21.[12] Borisov AB,Dedkov EI,Carlson BM.Differentiation of activated satellite cells in denervated muscle following single fusions in situ and in cell culture.Histochem Cell Biol.2005;124(1):13-23.[13] de Castro Rodrigues A,Andreo JC,de Mattos Rodrigues SP.Myonuclei and satellite cells in denervated rat muscles: an electron microscopy study.Microsurgery. 2006;26(5):396-398.[14] Williams AH,Liu N,van Rooij E,et al. MicroRNA control of muscle development and disease.Curr Opin Cell Biol. 2009;21(3):461-469.[15] O’Rourke JR,Georges SA,Seay HR,et al.Essential role forDicer during skeletal muscle development. Dev Biol. 2007;311:359-368.[16] Worton LE,Gardiner EM,Kwon RY,et al. Botulinum toxin A-induced muscle paralysis stimulates Hdac4 and differential miRNA expression. PLoS One.2018;13(11):e0207354. [17] Naya FJ,Olson E.MEF2: a transcriptional target for signaling pathways controlling skeletal muscle growth and differentiation.Curr Opin Cell Biol.1999;11(6):683-688.[18] Wu N,Gu T,Lu L,et al.Roles of miRNA-1 and miRNA-133 in the proliferation and differentiation of myoblasts in duck skeletal muscle. J Cell Physiol.2019;234(4):3490-3499.[19] 刘泽远,张文苹,黄强开,等.被动运动干预对大鼠失神经萎缩骨骼肌中miRNA-1表达和成肌细胞分化的影响[J].中国修复重建外科杂志, 2016, 30(5):612-618. [20] 谷造华,冯小宁,李永平,等.miRNA-1和miRNA-133在大鼠失神经腓肠肌中的变化规律[J].中华细胞与干细胞杂志:电子版, 2013,3(1):18-21.[21] Rao PK,Kumar RM,Farkhondeh M,et al.Myogenic factors that regulate expression of muscle-specific microRNAs. Proc Natl Acad Sci U S A. 2006;103(23):8721-8726.[22] Hsieh CH,Jeng SF,Wu CJ,et al.Altered expression of the microRNAS and their potential target genes in the soleus muscle after peripheral denervation and reinnervation in rats. J Trauma. 2011;70(2):472-480.[23] Ropero S,Esteller M.The role of histone deacetylases (HDACs) in human cancer.Mol Oncol. 2007;1(1):19-25.[24] Miska EA,Karlsson C,Langley E,et al.HDAC4 deacetylase associates with and represses the MEF2 tran-scription factor. Embo J. 1999;18(18): 5099-5107.[25] Braun T,Gautel M.Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nat Rev Mol Cell Biol. 2011; 12(6):349-361.[26] Clocchiatti A,Di Giorgio E,Demarchi F,et al.Beside the MEF2 axis: unconventional functions of HDAC4. Cell Signal.2013;25(1):269-276. |