Chinese Journal of Tissue Engineering Research ›› 2019, Vol. 23 ›› Issue (13): 2016-2021.doi: 10.3969/j.issn.2095-4344.1676
Previous Articles Next Articles
Human umbilical cord mesenchymal stem cells combined with immunotherapy for the treatment of type 1 diabetic mice
Guo Bo1, Liu Jia1, Cui Xiaolan2, Shi Han1, Zhang Sheyi1, Wang Jia1, Shan Xia1, Wang Yizhong1
- 1Department of Hematology and Endocrinology, Aerospace Clinical Medical College of Peking University, Beijing 100049, China; 2Pharmacology Laboratory, Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences, Beijing 100700, China
-
Revised:2018-12-24Online:2019-05-08Published:2019-05-08 -
Contact:Wang Yizhong, MD, Chief physician, Department of Hematology and Endocrinology, Aerospace Clinical Medical College of Peking University, Beijing 100049, China -
About author:Guo Bo, Master candidate, Department of Hematology and Endocrinology, Aerospace Clinical Medical College of Peking University, Beijing 100049, China -
Supported by:the Scientific Research Fund Project of the Aerospace Clinical Medical College of Peking University, No. YN201806 (to WYZ)
CLC Number:
Cite this article
Guo Bo, Liu Jia, Cui Xiaolan, Shi Han, Zhang Sheyi, Wang Jia, Shan Xia, Wang Yizhong. Human umbilical cord mesenchymal stem cells combined with immunotherapy for the treatment of type 1 diabetic mice[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(13): 2016-2021.
share this article
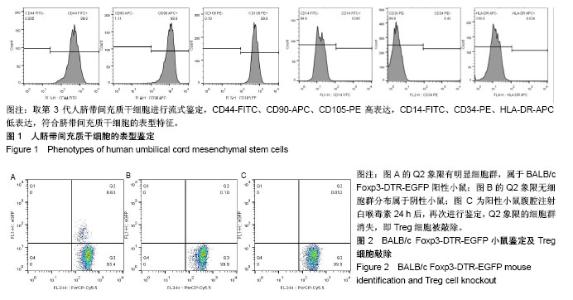
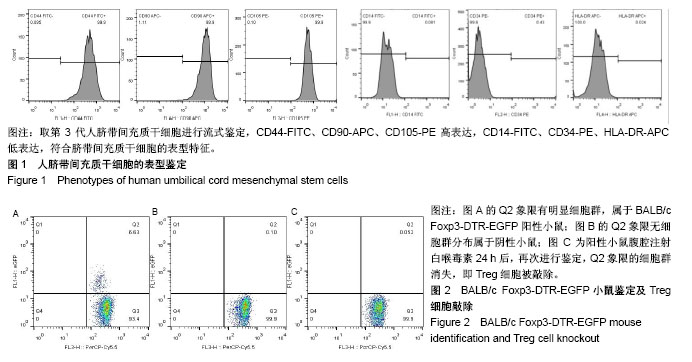
2.1 人脐带间充质干细胞的表型鉴定结果 如图1所示,流式细胞仪检测结果:CD44-FITC(99.9%)、CD90-APC (98.9%)、CD105-PE(99.9%)高表达,CD14-FITC (0.081%)、CD34-PE(0.43%)、HLA-DR-APC (0.024%)低表达,细胞表型符合人脐带间充质干细胞的表型特征。 2.2 BALB/c Foxp3-DTR-EGFP阳性小鼠的流式鉴定结果 取四五周龄小鼠,经眼眶采血进行流式鉴定,共鉴定115只小鼠,其中阳性小鼠59只,阳性率51.3%,图2A为阳性结果,图2B为阴性结果。 2.3 BALB/c Foxp3-DTR-EGFP阳性小鼠Treg细胞敲除 BALB/c Foxp3-DTR-EGFP阳性小鼠经腹腔注射白喉毒素后24 h,再次通过流式细胞仪对小鼠进行鉴定,结果如图2C所示,Q2象限细胞群消失,Treg细胞被成功敲除。"
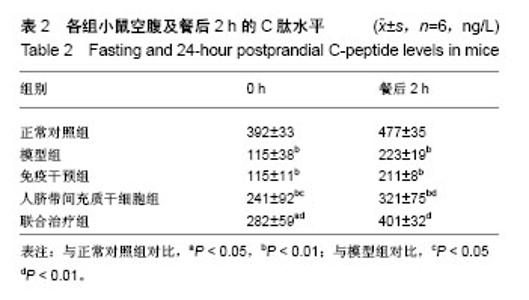
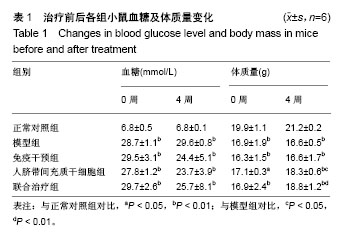
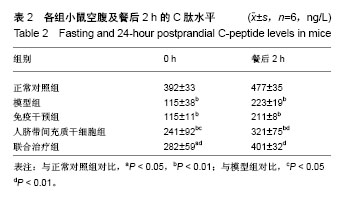
2.4.2 体质量变化 治疗后4周,正常对照组小鼠体质量明显增加,模型组小鼠体质量下降,两组对比差异有统计学意义(P < 0.01);免疫干预组、人脐带间充质干细胞组、联合治疗组小鼠体质量均有不同程度的增加,但与模型组对比,免疫干预组小鼠体质量变化不显著(P > 0.05);人脐带间充质干细胞组、联合治疗组小鼠体质量明显升高,与模型组对比差异有统计学意义(P < 0.05),其中联合治疗组体质量升高更加显著(P < 0.01),人脐带间充质干细胞组和联合治疗组小鼠体质量增量接近正常对照组水平。见表1。 2.4.3 C肽变化 治疗后4周,正常对照组小鼠C肽水平在合适范围内,模型组小鼠C肽水平明显下降,两组对比差异有统计学意义(P < 0.01);与模型组对比,免疫干预组小鼠血清C肽水平变化不显著(P > 0.05);人脐带间充质干细胞组及联合治疗组小鼠血清C肽水平显著升高,与模型组对比差异有统计学意义(P < 0.01);其中联合治疗组小鼠血清C肽水平改善最明显,接近正常对照组水平。见表2。"
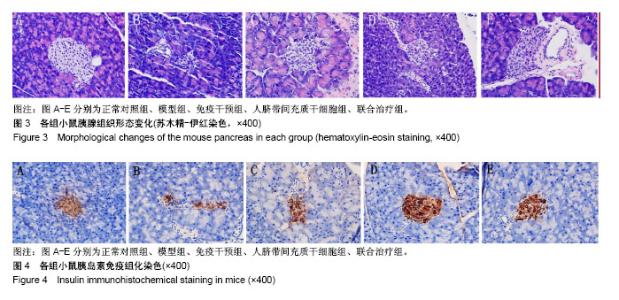
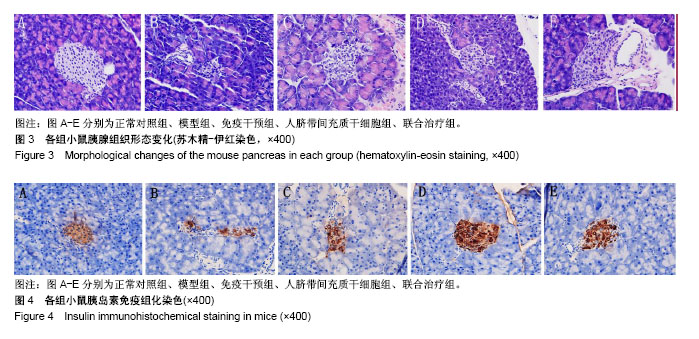
2.5 苏木精-伊红染色观察胰腺组织形态变化 正常对照组小鼠胰岛形态规则,呈圆形或者椭圆形细胞团,边界清楚,胰岛数及胰岛内细胞数量多,排列致密,胰岛细胞大小一致,细胞胞体饱满,胞质丰富,呈嗜酸性,核染色清晰呈卵圆形;模型组小鼠胰岛明显萎缩,轮廓不规则、扭曲、变形,边界模糊,胰岛数及胰岛内细胞数量明显减少,排列紊乱,分布稀疏;免疫干预组、人脐带间充质干细胞组、联合治疗组小鼠胰岛细胞受损程度较轻,胰岛轻度萎缩,胰岛形态较规则,界限清晰,胰岛内细胞数量较多,尤其是联合治疗组,部分小鼠胰岛形态完整接近正常组小鼠胰岛。见图3。 2.6 小鼠胰岛素免疫组织化学染色 正常对照组小鼠胰岛素染色阳性面积占满整个胰岛,分布均匀;模型组小鼠胰岛素染色阳性面积仅占胰岛小部分,且分布紊乱;免疫干预组、人脐带间充质干细胞组、联合治疗组小鼠胰岛素染色阳性面积占胰岛的大部分,且分布较均匀;与模型组对比,人脐带间充质干细胞组及联合治疗组小鼠胰岛素染色阳性面积变化显著,其中部分人脐带间充质干细胞组及联合治疗组小鼠胰岛素染色阳性面积接近正常组。见图4。"
| [1] Battaglia M, Atkinson MA. The Streetlight Effect in Type 1 Diabetes. Diabetes.2015;64(4):1081-1090.[2] Gülden E, Palm N, Herold KC. MAIT Cells: A Link between Gut Integrity and Type 1 Diabetes. Cell Metab. 2017;26(6):813-815.[3] Atkinson MA, von Herrath M, Powers AC, et al. Current concepts on the pathogenesis of type 1 diabetes-considerations for attempts to prevent and reverse the disease. Diabetes Care.2015;38(6):979-988.[4] Wang HS, Shyu JF, Shen WS,et al. Transplantation of insulin- producing cells derived from umbilical cord stromal mesenchymal stem cells to treat NOD mice. Cell Transplant. 2011;20(3):455-466.[5] Qu H, Liu X, Ni Y, et al. Laminin 411 acts as a potent inducer of umbilical cord mesenchymal stem cell differentiation into insulin-producing cells. J Transl Med.2014;12:135.[6] 申义,王意忠,时瀚,等.人脐带间充质干细胞分化胰岛样细胞过程中胰岛素和巢蛋白的表达[J]. 中国组织工程研究, 2016,20(50):7500-7506.[7] Kuchroo P, Dave V, Vijayan A, et al. Paracrine factors secreted by umbilical cord-derived mesenchymal stem cells induce angiogenesis in vitro by a VEGF-independent pathway.Stem Cells Dev. 2015;24(4): 437-450.[8] Shen C, Lie P, Miao T, et al. Conditioned medium from umbilical cord mesenchymal stem cells induces migration and angiogenesis. Mol Med Rep.2015;12(1):20-30.[9] Li T, Xia M, Gao Y, et al. Human umbilical cord mesenchymal stem cells: an overview of their potential in cell-based therapy. Expert Opin Biol Ther.2015;15(9):1293-1306.[10] Ding D, Chang Y, Shyu W, et al. Human umbilical cord mesenchymal stem cells: a new era for stem cell therapy. Cell Transplant. 2015; 24(3):339-347.[11] Lu LL, Liu YJ, Yang SG, et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis- supportive function and other potentials. Haematologica.2006; 91(8):1017-1026.[12] Wilckens T, De Rijk R. Glucocorticoids and immune function: unknown dimensions and new frontiers. Immunol Today. 1997; 18(9):418-424.[13] Londono P, Komura A, Hara N, et al. Brief dexamethasone treatment during acute infection prevents virus-induced autoimmune diabetes. Clin Immunol.2010;135(3):401-411.[14] Harrison LC, Wentworth JM, Zhang Y, et al. Antigen-based vaccination and prevention of type 1 diabetes. Curr Diab Rep.2013; 13(5):616-623.[15] Harrison LC, Honeyman MC, Steele CE, et al. Pancreatic beta-cell function and immune responses to insulin after administration of intranasal insulin to humans at risk for type 1 diabetes. Diabetes Care. 2004;27(10):2348-2355.[16] 于文龙,高宏,余霄龙,等. 脐带间充质干细胞移植治疗初发1型糖尿病[J]. 中国组织工程研究与临床康复, 2011,15(23):4363-4366.[17] 谭晓俊,王镇,李晓莹,等. 脐带间充质干细胞治疗1型糖尿病远期疗效观察三例报告[J]. 中华内分泌代谢杂志, 2016,32(12):1030-1032.[18] Geng S, Zhang H, Zhou X, et al. Diabetes tolerogenic vaccines targeting antigen-specific inflammation. Hum Vaccin Immunother. 2015;11(2): 522-530.[19] Campbell-Thompson ML, Atkinson MA, Butler AE, et al. The diagnosis of insulitis in human type 1 diabetes. Diabetologia. 2013; 56(11):2541-2543.[20] Krogvold L, Skog O, Sundstrom G, et al. Function of Isolated Pancreatic Islets From Patients at Onset of Type 1 Diabetes: Insulin Secretion Can Be Restored After Some Days in a Nondiabetogenic Environment In Vitro: Results From the DiViD Study. Diabetes.2015; 64(7):2506-2512.[21] Marhfour I, Lopez XM, Lefkaditis D, et al. Expression of endoplasmic reticulum stress markers in the islets of patients with type 1 diabetes. Diabetologia.2012;55(9):2417-2420.[22] Imai Y, Dobrian AD, Morris MA, et al. Lipids and immunoinflammatory pathways of beta cell destruction. Diabetologia.2016;59(4):673-678.[23] Grzesik WJ, Nadler JL, Machida Y, et al. Expression pattern of 12-lipoxygenase in human islets with type 1 diabetes and type 2 diabetes. J Clin Endocrinol Metab. 2015;100(3):E387-E395.[24] Willcox A, Richardson SJ, Bone AJ, et al. Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol. 2009; 155(2):173-181.[25] Visperas A, Vignali DA. Are Regulatory T Cells Defective in Type 1 Diabetes and Can We Fix Them?. J Immunol, 2016;197(10): 3762-3770.[26] D'Alessio FR, Tsushima K, Aggarwal NR, et al. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest. 2009;119(10): 2898-2913.[27] Liesz A, Suri-Payer E, Veltkamp C, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15(2):192-199.[28] Chen X, Zhou B, Li M, et al. CD4+CD25+FoxP3+ regulatory T cells suppress Mycobacterium tuberculosis immunity in patients with active disease. Clin Immunol.2007;123(1):50-59.[29] Qiao YC, Shen J, Hong XZ, et al. Changes of regulatory T cells, transforming growth factor-beta and interleukin-10 in patients with type 1 diabetes mellitus: A systematic review and meta-analysis. Clin Immunol. 2016;170:61-69.[30] Ghonaim MM, El-Edel RH, Kamal ES, et al. T-Regulatory Cell Subsets in Children with Type 1 Diabetes Mellitus: Relation to Control of the Disease. Endocr Metab Immune Disord Drug Targets. 2017; 17(3):238-245.[31] Lampeter EF, Signore A, Gale EA, et al. Lessons from the NOD mouse for the pathogenesis and immunotherapy of human type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1989;32(10): 703-708.[32] Lenzen S. Animal models of human type 1 diabetes for evaluating combination therapies and successful translation to the patient with type 1 diabetes. Diabetes Metab Res Rev. 2017;33(7). doi: 10.1002/dmrr.2915.[33] Geng S, Zhang H, Zhou X, et al. Diabetes tolerogenic vaccines targeting antigen-specific inflammation. Hum Vaccin Immunother. 2015;11(2): 522-530.[34] Zipris D. Innate immunity in type 1 diabetes. Diabetes Metab Res Rev. 2011;27(8): 824-829.[35] Hu J, Wang Y, Wang F, et al. Effect and mechanisms of human Wharton??s jelly-derived mesenchymal stem cells on type 1 diabetes in NOD model. Endocrine. 2015;48(1):124-134.[36] Wang D, Chen K, Du W T, et al. CD14+ monocytes promote the immunosuppressive effect of human umbilical cord matrix stem cells. Exp Cell Res. 2010;316(15): 2414-2423.[37] Ribeiro A, Laranjeira P, Mendes S, et al. Mesenchymal stem cells from umbilical cord matrix, adipose tissue and bone marrow exhibit different capability to suppress peripheral blood B, natural killer and T cells.Stem Cell Res Ther. 2013;4(5):125.[38] Tang RJ, Shen SN, Zhao XY, et al. Mesenchymal stem cells-regulated Treg cells suppress colitis-associated colorectal cancer. Stem Cell Res Ther. 2015;6:71.[39] Wang D, Huang S, Yuan X, et al. The regulation of the Treg/Th17 balance by mesenchymal stem cells in human systemic lupus erythematosus. Cell Mol Immunol. 2017;14(5):423-431.[40] Montanucci P, Alunno A, Basta G, et al. Restoration of t cell substes of patients with type 1 diabetes mellitus by microencapsulated human umbilical cord Wharton jelly-derived mesenchymal stem cells: An in vitro study. Clin Immunol.2016;163:34-41.[41] 赵子粼,叶常青,罗建春,等. 脐带间充质干细胞移植对心力衰竭大鼠外周血CD_4~+CD_(25)~+Foxp_3~+Treg细胞表达的影响[J]. 山东医药, 2015,55(41):13-15.[42] El-Hossary N, Hassanein H, El Ghareeb AW, et al. Intravenous vs intraperitoneal transplantation of umbilical cord mesenchymal stem cells from Wharton??s jelly in the treatment of streptozotocin- induced diabetic rats. Diabetes Res Clin Pract. 2016; 21:102-111.[43] Sun X, Hao H, Han Q, et al. Human umbilical cord-derived mesenchymal stem cells ameliorate insulin resistance by suppressing NLRP3 inflammasome-mediated inflammation in type 2 diabetes rats. Stem Cell Res Ther.2017;8(1):241. |
| [1] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [2] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [3] | Zhang Xiumei, Zhai Yunkai, Zhao Jie, Zhao Meng. Research hotspots of organoid models in recent 10 years: a search in domestic and foreign databases [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1249-1255. |
| [4] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [5] | Shi Yangyang, Qin Yingfei, Wu Fuling, He Xiao, Zhang Xuejing. Pretreatment of placental mesenchymal stem cells to prevent bronchiolitis in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 991-995. |
| [6] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [7] | Fan Quanbao, Luo Huina, Wang Bingyun, Chen Shengfeng, Cui Lianxu, Jiang Wenkang, Zhao Mingming, Wang Jingjing, Luo Dongzhang, Chen Zhisheng, Bai Yinshan, Liu Canying, Zhang Hui. Biological characteristics of canine adipose-derived mesenchymal stem cells cultured in hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1002-1007. |
| [8] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [9] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [10] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| [11] | Duan Liyun, Cao Xiaocang. Human placenta mesenchymal stem cells-derived extracellular vesicles regulate collagen deposition in intestinal mucosa of mice with colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1026-1031. |
| [12] | Pei Lili, Sun Guicai, Wang Di. Salvianolic acid B inhibits oxidative damage of bone marrow mesenchymal stem cells and promotes differentiation into cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1032-1036. |
| [13] | Guan Qian, Luan Zuo, Ye Dou, Yang Yinxiang, Wang Zhaoyan, Wang Qian, Yao Ruiqin. Morphological changes in human oligodendrocyte progenitor cells during passage [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1045-1049. |
| [14] | Wang Zhengdong, Huang Na, Chen Jingxian, Zheng Zuobing, Hu Xinyu, Li Mei, Su Xiao, Su Xuesen, Yan Nan. Inhibitory effects of sodium butyrate on microglial activation and expression of inflammatory factors induced by fluorosis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1075-1080. |
| [15] | Wang Xianyao, Guan Yalin, Liu Zhongshan. Strategies for improving the therapeutic efficacy of mesenchymal stem cells in the treatment of nonhealing wounds [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1081-1087. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||