Chinese Journal of Tissue Engineering Research ›› 2019, Vol. 23 ›› Issue (1): 68-73.doi: 10.3969/j.issn.2095-4344.1527
Previous Articles Next Articles
Effect of adipose-derived mesenchymal stem cell exosomes on the proliferation and migration of keratinocytes and the underlying mechanisms
Tian Xinli, Jiang Bo, Yan Hong
- Department of Plastic and Burn Surgery, Affiliated Hospital of Southwest Medical University, Luzhou 646000, Sichuan Province, China
-
Revised:2018-09-27Online:2019-01-08Published:2018-11-28 -
Contact:Yan Hong, MD, Chief physician, Department of Plastic and Burn Surgery, Affiliated Hospital of Southwest Medical University, Luzhou 646000, Sichuan Province, China -
About author:Tian Xinli, Master, Physician, Department of Plastic and Burn Surgery, Affiliated Hospital of Southwest Medical University, Luzhou 646000, Sichuan Province, China
CLC Number:
Cite this article
Tian Xinli, Jiang Bo, Yan Hong. Effect of adipose-derived mesenchymal stem cell exosomes on the proliferation and migration of keratinocytes and the underlying mechanisms[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(1): 68-73.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
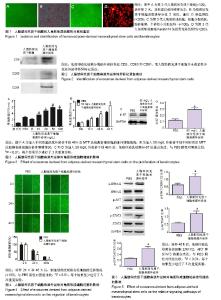
2.1 人ADSCs和人角质形成细胞的分离和鉴定结果 分离出的ADSCs呈成纤维样形状,且增殖率高,见图1A。为了进一步确定分离细胞即为ADSCs,对细胞进行成脂诱导分化培养2周,油红O染色呈阳性,见图1B。 第3代人角质形成细胞均分裂活跃,胞体饱满,个体较小呈骰子状,细胞形态均一性好,见图1C。进一步通过角质形成细胞特异标记蛋白Keratin14免疫荧光染色鉴定,提示所分离的细胞为角质形成细胞,见图1D。 2.2 人ADSCs来源外泌体的鉴定结果 利用超速离心法分离ADSCs来源外泌体,通过免疫印迹法检测外泌体特异标记蛋白CD9、CD63和CD81表达,见图2。分离的外泌体表达CD9、CD63和CD81,而ADSCs并未或者很少表达外泌体特异标记蛋白,表明成功分离出ADSCs来源外泌体。 2.3 人ADSCs来源外泌体对角质形成细胞增殖的影响 角质形成细胞分别加入不同质量浓度(10,20,50,100 mg/L) ADSCs来源外泌体培养48 h后,采用MTT法检测各组细胞的体外增殖情况,见图3A,在加入外泌体后,角质形成细胞的增殖速度显著高于PBS阴性对照组,且呈浓度依赖性增长,在质量浓度为50 mg/L时增殖最显著;随后加入50 mg/L ADSCs来源外泌体培养0,12,24,48 h,采用MTT法检测各组细胞的体外增殖情况,见图3B,外泌体能够显著促进角质形成细胞增殖,且随着时间的增加而增加。 为进一步验证上述实验结果,角质形成细胞分别加入PBS和50 mg/L ADSCs来源外泌体培养48 h后,采用免疫印迹法检测细胞增殖标记物ki-67的表达水平,得到与MTT法相似的结果,见图3C和3D。以上结果提示,人ADSCs来源外泌体可以显著促进角质形成细胞在体外的增殖。 2.4 人ADSCs来源外泌体对角质形成细胞迁移的影响 角质形成细胞加入50 mg/L ADSCs来源外泌体培养,利用细胞划痕法观察24 h和48 h后各组细胞的迁移情况,见图4,在加入外泌体后,角质形成细胞的迁移速度显著高于PBS阴性对照组,且随着时间的增加而增加。以上结果提示,外泌体可以显著促进角质形成细胞的迁移。 2.5 人ADSCs来源外泌体对角质形成细胞相关信号通路蛋白的影响 研究发现,ERK1/2、AKT和STAT3信号通路在创面愈合过程中起到重要的作用,包括细胞增殖、迁移和血管生成等[26]。因此,实验检测ADSCs来源外泌体是否通过ERK1/2、AKT和STAT3信号通路进而影响其增殖与迁移,见图5。角质形成细胞加入50 mg/L ADSCs来源外泌体培养48 h后,ERK1/2、AKT和STAT3的磷酸化水平显著高于PBS阴性对照组。以上结果提示,外泌体可能通过ERK1/2、AKT和STAT3信号通路进而影响其增殖与迁移。"
| [1] Li W, Wu X, Gao C.Ten-year epidemiological study of chemical burns in Jinshan, Shanghai, PR China.Burns. 2013;39(7):1468-1473. [2] Blais M, Parenteau-Bareil R, Cadau S, et al. Concise review: tissue-engineered skin and nerve regeneration in burn treatment.Stem Cells Transl Med. 2013;2(7):545-551. [3] Spronk I, Legemate CM, Dokter J, et al. Predictors of health-related quality of life after burn injuries: a systematic review.Crit Care. 2018;22(1):160. [4] Mustoe TA, O'Shaughnessy K, Kloeters O.Chronic wound pathogenesis and current treatment strategies: a unifying hypothesis. PlastReconstr Surg. 2006;117(7 Suppl):35S-41S. [5] Raja, Sivamani K, Garcia MS,et al. Wound re-epithelialization: modulating keratinocyte migration in wound healing.Front Biosci. 2007;12:2849-2868. [6] Moura LI, Cruz MT, Carvalho E.The effect of neurotensin in human keratinocytes--implication on impaired wound healing in diabetes. ExpBiol Med (Maywood). 2014;239(1):6-12. [7] Taylor, Richard MI. Identification of M-CSF independent mechanisms of human osteoclast formation and analysis of the cellular and molecular mechanisms involved in tumourosteolysis. Voprosy Medit?sinsko? Khimii. 2011;11(5):22-26. [8] Pesce M, Patruno A, Speranza L, et al. Extremely low frequency electromagnetic field and wound healing: implication of cytokines as biological mediators.Eur Cytokine Netw. 2013;24(1):1-10. [9] Baum CL, Arpey CJ.Normal cutaneous wound healing: clinical correlation with cellular and molecular events. Dermatol Surg. 2005; 31(6):674-686. [10] Galiano RD, Tepper OM, Pelo CR, et al. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells.Am J Pathol. 2004;164(6):1935-1947. [11] Jhamb S, Vangaveti VN, Malabu UH.Genetic and molecular basis of diabetic foot ulcers: Clinical review.J Tissue Viability. 2016;25(4):229-236. [12] Lerman OZ, Galiano RD, Armour M, et al. Cellular dysfunction in the diabetic fibroblast: impairment in migration, vascular endothelial growth factor production, and response to hypoxia.Am J Pathol. 2003; 162(1):303-312. [13] Teixeira FG, Carvalho MM, Neves-Carvalho A, et al. Secretome of mesenchymal progenitors from the umbilical cord acts as modulator of neural/glial proliferation and differentiation.Stem Cell Rev. 2015;11(2): 288-297. [14] Wilson JJ, Foyle KL, Foeng J, et al. Redirecting adult mesenchymal stromal cells to the brain: a new approach for treating CNS autoimmunity and neuroinflammation. Immunol Cell Biol. 2018;96(4):347-357. [15] Lee SH, Lee JH, Cho KH.Effects of Human Adipose-derived Stem Cells on Cutaneous Wound Healing in Nude Mice.Ann Dermatol. 2011;23(2):150-155. [16] Nambu M, Kishimoto S, Nakamura S, et al. Accelerated wound healing in healing-impaired db/db mice by autologous adipose tissue-derived stromal cells combined with atelocollagen matrix.Ann Plast Surg. 2009; 62(3):317-321. [17] Lee SH, Jin SY, Song JS, et al. Paracrine effects of adipose-derived stem cells on keratinocytes and dermal fibroblasts.Ann Dermatol. 2012; 24(2):136-143. [18] Vlassov AV, Magdaleno S, Setterquist R, et al. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820(7):940-948. [19] Aryani A, Denecke B.Exosomes as a Nanodelivery System: a Key to the Future of Neuromedicine. MolNeurobiol. 2016;53(2):818-834. [20] Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells.Nat Cell Biol. 2007;9(6):654-659. [21] Li Z, Wang Y, Xiao K, et al. Emerging Role of Exosomes in the Joint Diseases.Cell Physiol Biochem. 2018;47(5):2008-2017. [22] Schorey JS, Bhatnagar S.Exosome function: from tumor immunology to pathogen biology.Traffic. 2008;9(6):871-881. [23] Zhang W, Zhou X, Yao Q, et al. HIF-1-mediated production of exosomes during hypoxia is protective in renal tubular cells.Am J Physiol Renal Physiol. 2017;313(4):F906-F913. [24] Li T, Yan Y, Wang B, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis.Stem Cells Dev. 2013;22(6):845-854. [25] Zhang Y, Chopp M, Liu XS, et al. Exosomes Derived from Mesenchymal Stromal Cells Promote Axonal Growth of Cortical Neurons. Mol Neurobiol. 2017;54(4):2659-2673. [26] Akita S, Akino K, Hirano A.Basic Fibroblast Growth Factor in Scarless Wound Healing.Adv Wound Care (New Rochelle). 2013;2(2):44-49. [27] Bruno S, Grange C, Deregibus MC, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury.J Am Soc Nephrol. 2009;20(5):1053-1067. [28] Vella LJ, Sharples RA, Nisbet RM, et al.The role of exosomes in the processing of proteins associated with neurodegenerative diseases. Eur Biophys J. 2008;37(3):323-332. [29] Herrera MB, Fonsato V, Gatti S, et al. Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats.J Cell Mol Med. 2010;14(6B):1605-1618. [30] Peinado H, Ale?kovi? M, Lavotshkin S, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET.Nat Med. 2012;18(6):883-891. [31] Atay S, Banskota S, Crow J, et al. Oncogenic KIT-containing exosomes increase gastrointestinal stromal tumor cell invasion.Proc Natl Acad Sci U S A. 2014;111(2):711-716. [32] Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury.Stem Cell Res. 2010;4(3):214-222. [33] Xin H, Li Y, Cui Y, et al. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats.J Cereb Blood Flow Metab. 2013;33(11):1711-1715. [34] Zhu YG, Feng XM, Abbott J, et al. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice.Stem Cells. 2014;32(1):116-125. [35] Shabbir A, Cox A, Rodriguez-Menocal L, et al. Mesenchymal Stem Cell Exosomes Induce Proliferation and Migration of Normal and Chronic Wound Fibroblasts, and Enhance Angiogenesis In Vitro.Stem Cells Dev. 2015;24(14):1635-1647. [36] Nishikai-Yan Shen T, Kanazawa S, Kado M, et al. Interleukin-6 stimulates Akt and p38 MAPK phosphorylation and fibroblast migration in non-diabetic but not diabetic mice. PLoS One. 2017;12(5):e0178232. [37] Wang C, Zhang Z, Xu T, et al. Upregulating mTOR/ERK signaling with leonurine for promoting angiogenesis and tissue regeneration in a full-thickness cutaneous wound model.Food Funct. 2018;9(4):2374-2385. [38] Sun Q, Rabbani P, Takeo M, et al. Dissecting Wnt Signaling for Melanocyte Regulation during Wound Healing.J Invest Dermatol. 2018;138(7):1591-1600. [39] Hsiao CM, Wu YS, Nan FH, et al. Immunomodulator 'mushroom beta glucan' induces Wnt/β catenin signalling and improves wound recovery in tilapia and rat skin: a histopathological study.Int Wound J. 2016;13(6):1116-1128. [40] Rosca AM, Rayia DM, Tutuianu R.Emerging Role of Stem Cells - Derived Exosomes as Valuable Tools for Cardiovascular Therapy.Curr Stem Cell Res Ther. 2017;12(2):134-138. |
| [1] | Pu Rui, Chen Ziyang, Yuan Lingyan. Characteristics and effects of exosomes from different cell sources in cardioprotection [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 1-. |
| [2] | Lin Qingfan, Xie Yixin, Chen Wanqing, Ye Zhenzhong, Chen Youfang. Human placenta-derived mesenchymal stem cell conditioned medium can upregulate BeWo cell viability and zonula occludens expression under hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(在线): 4970-4975. |
| [3] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [4] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [5] | Shi Yangyang, Qin Yingfei, Wu Fuling, He Xiao, Zhang Xuejing. Pretreatment of placental mesenchymal stem cells to prevent bronchiolitis in mice [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 991-995. |
| [6] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [7] | Fan Quanbao, Luo Huina, Wang Bingyun, Chen Shengfeng, Cui Lianxu, Jiang Wenkang, Zhao Mingming, Wang Jingjing, Luo Dongzhang, Chen Zhisheng, Bai Yinshan, Liu Canying, Zhang Hui. Biological characteristics of canine adipose-derived mesenchymal stem cells cultured in hypoxia [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1002-1007. |
| [8] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [9] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [10] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| [11] | Duan Liyun, Cao Xiaocang. Human placenta mesenchymal stem cells-derived extracellular vesicles regulate collagen deposition in intestinal mucosa of mice with colitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1026-1031. |
| [12] | Pei Lili, Sun Guicai, Wang Di. Salvianolic acid B inhibits oxidative damage of bone marrow mesenchymal stem cells and promotes differentiation into cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1032-1036. |
| [13] | Li Cai, Zhao Ting, Tan Ge, Zheng Yulin, Zhang Ruonan, Wu Yan, Tang Junming. Platelet-derived growth factor-BB promotes proliferation, differentiation and migration of skeletal muscle myoblast [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1050-1055. |
| [14] | Liu Cong, Liu Su. Molecular mechanism of miR-17-5p regulation of hypoxia inducible factor-1α mediated adipocyte differentiation and angiogenesis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1069-1074. |
| [15] | Wang Xianyao, Guan Yalin, Liu Zhongshan. Strategies for improving the therapeutic efficacy of mesenchymal stem cells in the treatment of nonhealing wounds [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1081-1087. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||