Chinese Journal of Tissue Engineering Research ›› 2019, Vol. 23 ›› Issue (2): 204-210.doi: 10.3969/j.issn.2095-4344.1508
Previous Articles Next Articles
Preparation and characterization of novel porous polymethyl methacrylate bone cements
Gao Shan1, Zhou Fang1, Lü Yang1, Yuan Liang1, Li Ailing2, Qiu Dong2
- 1Department of Orthopedics, Peking University Third Hospital, Beijing 100191, China; 2Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China
-
Received:2018-09-26Online:2019-01-18Published:2019-01-18 -
Contact:Zhou Fang, Chief physician, Professor, Department of Orthopedics, Peking University Third Hospital, Beijing 100191, China -
About author:Gao Shan, Doctorate candidate, Department of Orthopedics, Peking University Third Hospital, Beijing 100191, China -
Supported by:the National Natural Science Foundation of China, No. 51473004 (to ZF)
CLC Number:
Cite this article
Gao Shan, Zhou Fang, Lü Yang, Yuan Liang, Li Ailing, Qiu Dong. Preparation and characterization of novel porous polymethyl methacrylate bone cements[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(2): 204-210.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
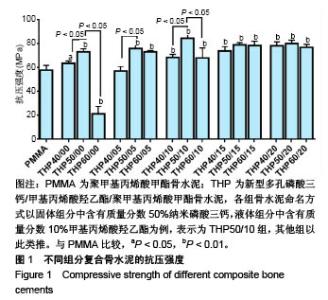
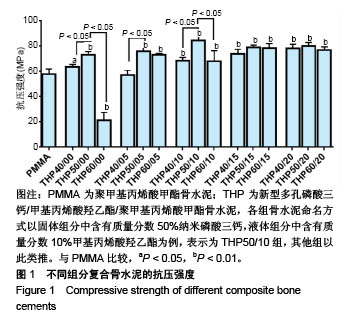
2.1 各组复合骨水泥的抗压强度 各组分骨水泥的抗压强度结果,见图1。 与对照组比较,THP60/00组抗压模量降低(P < 0.01),其他组抗压模量均升高,除其中的THP40/05组抗压模量与对照组相比差异无显著性意义外(P > 0.05),其余比较均有显著性意义(P < 0.05或P < 0.01)。 在实验组组内,当HEMA比例一定时(0%,5%,10%),纳米磷酸三钙质量分数为50%组分的抗压模量优于40%、60%组分(P < 0.05);当HEMA比例≥15%时,纳米磷酸三钙比例变化对复合骨水泥的抗压模量无明显影响(P > 0.05)。 在实验组组内,当纳米磷酸三钙比例一定时,抗压模量在一定范围内随着HEMA比例(0%-15%)的增高逐渐升高;当HEMA比例≥15%时,HEMA比例增高对复合骨水泥的抗压模量无明显影响(P > 0.05)。实验表明磷酸三钙和HEMA的加入,可一定范围内增强复合骨水泥的抗压性能。以PMMA组为参照,其中THP50/00组、THP50/05组、THP50/10组为优势组分。"
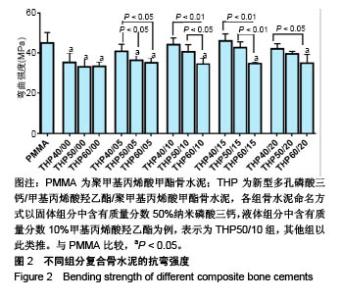
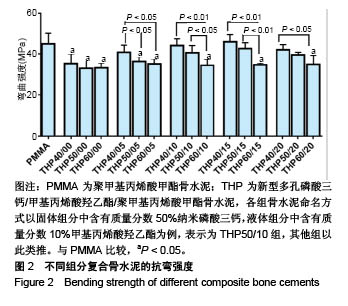
2.2 各组复合骨水泥的抗弯强度 各组分骨水泥的抗弯强度结果,见图2。 与对照组相比,THP40/00组、THP50/00组、THP50/05组及所有磷酸三钙质量分数为60%组分的复合骨水泥抗弯强度明显下降,呈线性关系(P < 0.05)。 在实验组组内,当HEMA比例一定时,随着纳米磷酸三钙比例的增加,复合骨水泥的抗弯强度逐渐降低;在所有含有HEMA组分的复合骨水泥中,40%纳米磷酸三钙组分的抗弯强度高于60%纳米磷酸三钙组分(P < 0.05);当HEMA比例为10%,15%时,50%纳米磷酸三钙组分的抗弯强度高于60%纳米磷酸三钙组分(P < 0.05)。 在实验组组内,当磷酸三钙比例一定时(40%,50%),随着HEMA比例(0%-15%)的增加,复合骨水泥的抗弯强度逐渐上升;当HEMA比例≥15%时,复合骨水泥的抗弯强度下降,并且THP40/20组、THP50/20组的抗弯强度分别小于THP40/15组、THP50/15组(P < 0.05);在60%磷酸三钙组分中,复合骨水泥的抗弯强度并没有随着HEMA比例的变化发生明显变化,稳定在35 MPa左右。实验提示磷酸三钙的加入,可显著降低复合骨水泥的抗弯性能,但加入HEMA后可在一定范围内加强复合骨水泥的抗弯性能。以对照组为参照,进一步筛选,发现THP50/10组为优势组分。"
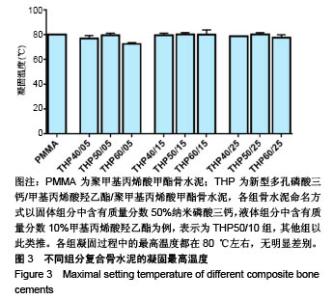
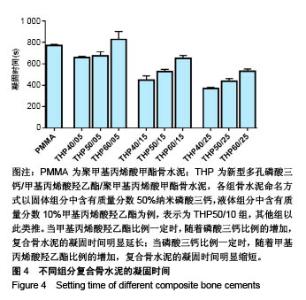
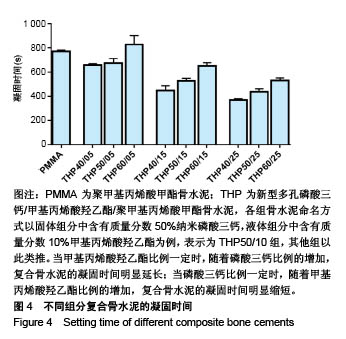
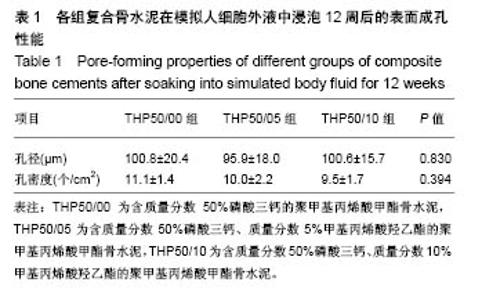
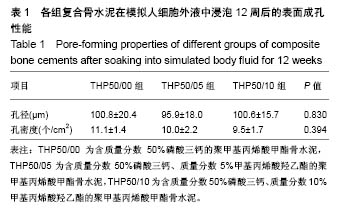
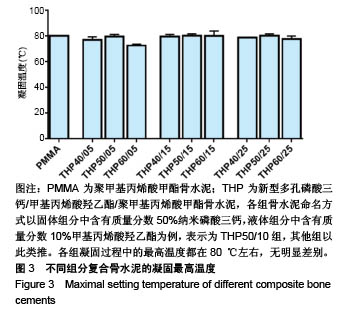
2.3 各组复合骨水泥的凝固最高温度和凝固时间 各组复合骨水泥的凝固最高温度结果,见图3。各实验组凝固过程中的最高温度都在80 ℃左右,与对照组无明显差别,并没有随着磷酸三钙和HEMA比例的变化发生明显变化。 各组复合骨水泥的凝固时间结果,见图4。与凝固最高温度不同,复合骨水泥的凝固时间明显受到磷酸三钙、HEMA比例的影响:当HEMA比例一定时,随着磷酸三钙比例的增加,复合骨水泥的凝固时间明显延长;当磷酸三钙比例一定时,随着HEMA比例的增加,复合骨水泥的凝固时间明显缩短。根据ISO5833标准对丙烯酸类骨水泥凝固温度为(90±5) ℃[22],凝固时间6.5-15 min之间的要求,THP50/10组满足该要求。"
| [1] CHARNLEY J.Anchorage of the femoral head prosthesis to the shaft of the femur. J Bone Joint Surg Br.1960;42-B:28-30.[2] Mahon OR,O'Hanlon S,Cunningham CC,et al.Orthopaedic implant materials drive M1 macrophage polarization in a spleen tyrosine kinase- and mitogen-activated protein kinase-dependent manner. Acta Biomater.2018;65:426-435.[3] Gao X,Ge J,Li W,et al. LncRNA KCNQ1OT1 ameliorates particle-induced osteolysis through inducing macrophage polarization by inhibiting miR-21a-5p.Biol Chem. 2018; 399(4): 375-386.[4] Antonios JK,Yao Z,Li C,et al. Macrophage polarization in response to wear particles in vitro. Cell Mol Immunol. 2013;10(6): 471-482. [5] Kim W,Yoon PW,Kwak HS,et al.Primary hybrid THA using a polymethyl methacrylate-precoated stem: A single-center experience with a 10-year minimum follow-up. J Biomed Mater Res B Appl Biomater. 2017;105(5):1300-1306.[6] Ayre WN,Denyer SP,Evans SL.Ageing and moisture uptake in polymethyl methacrylate (PMMA) bone cements.J Mech Behav Biomed Mater.2014;32:76-88. [7] Dall'Oca C,Maluta T,Cavani F,et al. The biocompatibility of porous vs non-porous bone cements: a new methodological approach. Eur J Histochem.2014;58(2):2255. [8] Yang J,Zhang K,Zhang S,et al.Preparation of calcium phosphate cement and polymethyl methacrylate for biological composite bone cements.Med Sci Monit.2015;21:1162-1172.[9] Lye KW,Tideman H,Wolke JC,et al.Biocompatibility and bone formation with porous modified PMMA in normal and irradiated mandibular tissue.Clin Oral Implants Res.2013;24 Suppl A 100: 100-109.[10] Sa Y,Yu N,Wolke JGC,et al.Bone Response to Porous Poly(methyl methacrylate) Cement Loaded with Hydroxyapatite Particles in a Rabbit Mandibular Model.Tissue Eng Part C Methods.2017;23(5):262-273.[11] Sa Y, Yang F,de Wijn JR, et al. Physicochemical properties and mineralization assessment of porous polymethylmethacrylate cement loaded with hydroxyapatite in simulated body fluid. Mater Sci Eng C Mater Biol Appl. 2016;61:190-198. [12] Wang L,Yoon DM,Spicer PP,et al.Characterization of porous polymethylmethacrylate space maintainers for craniofacial reconstruction.J Biomed Mater Res B Appl Biomater. 2013;101(5): 813-825.[13] Lewis G. Properties of acrylic bone cement: state of the art review. J Biomed Mater Res. 1997;38(2):155-182.[14] 张森林.磷酸钙骨水泥的研究进展[J].医学研究生学报, 2003,16(1): 62-63. [15] 陈浩东,姚金凤,梁志刚.磷酸钙的固有骨诱导性及其应用[J].中国组织工程研究,2016,20(25):3785-3792. [16] Li Y,Jiang T,Zheng L,et al.Osteogenic differentiation of mesenchymal stem cells (MSCs) induced by three calcium phosphate ceramic (CaP) powders: A comparative study.Mater Sci Eng C Mater Biol Appl. 2017;80:296-300. [17] 尚希福,汤亭亭,戴尅戎.TCP-PMMA骨水泥-骨界面强度变化的研究[J].医用生物力学,2006,21(2):111-114. [18] Liu Z, Tang Y, Kang T, et al. Synergistic effect of HA and BMP-2 mimicking peptide on the bioactivity of HA/PMMA bone cement.Colloids Surf B Biointerfaces.2015;131:39-46. [19] Lewis G.Properties of nanofiller-loaded poly (methyl methacrylate) bone cement composites for orthopedic applications: a review.J Biomed Mater Res B Appl Biomater. 2017;105(5):1260-1284. [20] Wolf-Brandstetter C,Roessler S,Storch S,et al.Physicochemical and cell biological characterization of PMMA bone cements modified with additives to increase bioactivity. J Biomed Mater Res B Appl Biomater. 2013;101(4):599-609[21] Klaus-DieterKuhn.PMMA骨水泥[M].张克,吕维加译.北京:北京大学医学出版社,2015.[22] 行业标准YY0459-2003/ISO5833:2002丙烯酸类树脂骨水泥:国家食品药品监督管理局,2013.[23] ISO 10993-11:1994 , Biological evaluation of medical devices-Part 11:Tests for systemic toxicity.2003.[24] Langer R,Vacanti JP.Tissue engineering. Science. 1993;260 (5110):920-926. [25] Fottner A,Nies B,Kitanovic D,et al.Performance of bioactive PMMA-based bone cement under load-bearing conditions: an in vivo evaluation and FE simulation. J Mater Sci Mater Med. 2016; 27(9):138.[26] Verburg H, van de Ridder LC, Verhoeven VW, et al. Validation of a measuring technique with computed tomography for cement penetration into trabecular bone underneath the tibial tray in total knee arthroplasty on a cadaver model. BMC Med Imaging 2014; 14:29. [27] Van Susante JLC,Verdonschot N,Bom LPA,et al.Lessons learnt from early failure of a patient trial with a polymer-on-polymer resurfacing hip arthroplasty.Acta Orthop. 2018;89(1):59-65.[28] Chen C,Li D,Wang Z,et al.Safety and Efficacy Studies of Vertebroplasty, Kyphoplasty, and Mesh-Container-Plasty for the Treatment of Vertebral Compression Fractures: Preliminary Report. PLoS One.2016;11(3):e0151492. [29] Wang H,Sribastav SS,Ye F,et al.Comparison of Percutaneous Vertebroplasty and Balloon Kyphoplasty for the Treatment of Single Level Vertebral Compression Fractures: A Meta-analysis of the Literature. Pain Physician. 2015;18(3):209-222.[30] Chandra W,Prasad BC,Jagadeesh MA,et al.Segmental polymethylmethacrylate-augmented fenestrated pedicle screw fixation for lumbar spondylolisthesis in patients with osteoporosis - A case series and review of literature.Neurol India. 2017;65(1): 89-95. [31] Kim JG,Jin YJ,Chung SK,et al.Unilateral augmented pedicle screw fixation for foraminal stenosis. J Korean Neurosurg Soc. 2009;46(1):5-10. [32] Yimin Y,Zhiwei R,Wei M,et al.Current status of percutaneous vertebroplasty and percutaneous kyphoplasty--a review.Med Sci Monit.2013;19:826-836. [33] Wegener B,Zolyniak N,Gülecyüz MF,et al. Heat distribution of polymerisation temperature of bone cement on the spinal canal during vertebroplasty.Int Orthop.2012;36(5):1025-1030.[34] McMahon S,Hawdon G,Baré J,et al.In vivo response of bone defects filled with PMMA in an ovine model.Hip Int. 2011;21(5): 616-622. [35] Xu HH,Burguera EF,Carey LE.Strong, macroporous, and in situ-setting calcium phosphate cement-layered structures. Biomaterials.2007;28(26):3786-3796. [36] Tsuruga E,Takita H,Itoh H,et al.Pore size of porous hydroxyapatite as the cell-substratum controls BMP-induced osteogenesis.J Biochem.1997;121(2):317-324. [37] Moreau MF,Chappard D,Lesourd M,et al.Free radicals and side products released during methylmethacrylate polymerization are cytotoxic for osteoblastic cells.J Biomed Mater Res. 1998;40(1): 124-131.[38] Bettencourt A,Calado A,Amaral J, et al.In vitro release studies of methylmethacrylate liberation from acrylic cement powder. Int J Pharm.2000;197(1-2):161-168. [39] Hoess A,López A,Engqvist H, et al.Comparison of a quasi-dynamic and a static extraction method for the cytotoxic evaluation of acrylic bone cements. Mater Sci Eng C Mater Biol Appl.2016;62:274-282.[40] 郭新辉,吕扬阳,范积平,等.磷酸钙与聚甲基丙烯酸甲酯制备复合型骨水泥的生物安全性研究[J].中国骨与关节损伤杂志, 2016,31(5): 506-510. |
| [1] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [2] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [3] | Xu Dongzi, Zhang Ting, Ouyang Zhaolian. The global competitive situation of cardiac tissue engineering based on patent analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 807-812. |
| [4] | Wu Zijian, Hu Zhaoduan, Xie Youqiong, Wang Feng, Li Jia, Li Bocun, Cai Guowei, Peng Rui. Three-dimensional printing technology and bone tissue engineering research: literature metrology and visual analysis of research hotspots [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 564-569. |
| [5] | Chang Wenliao, Zhao Jie, Sun Xiaoliang, Wang Kun, Wu Guofeng, Zhou Jian, Li Shuxiang, Sun Han. Material selection, theoretical design and biomimetic function of artificial periosteum [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 600-606. |
| [6] | Liu Fei, Cui Yutao, Liu He. Advantages and problems of local antibiotic delivery system in the treatment of osteomyelitis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 614-620. |
| [7] | Li Xiaozhuang, Duan Hao, Wang Weizhou, Tang Zhihong, Wang Yanghao, He Fei. Application of bone tissue engineering materials in the treatment of bone defect diseases in vivo [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 626-631. |
| [8] | Zhang Zhenkun, Li Zhe, Li Ya, Wang Yingying, Wang Yaping, Zhou Xinkui, Ma Shanshan, Guan Fangxia. Application of alginate based hydrogels/dressings in wound healing: sustained, dynamic and sequential release [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 638-643. |
| [9] | Chen Jiana, Qiu Yanling, Nie Minhai, Liu Xuqian. Tissue engineering scaffolds in repairing oral and maxillofacial soft tissue defects [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 644-650. |
| [10] | Xing Hao, Zhang Yonghong, Wang Dong. Advantages and disadvantages of repairing large-segment bone defect [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 426-430. |
| [11] | Chen Siqi, Xian Debin, Xu Rongsheng, Qin Zhongjie, Zhang Lei, Xia Delin. Effects of bone marrow mesenchymal stem cells and human umbilical vein endothelial cells combined with hydroxyapatite-tricalcium phosphate scaffolds on early angiogenesis in skull defect repair in rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3458-3465. |
| [12] | Wang Hao, Chen Mingxue, Li Junkang, Luo Xujiang, Peng Liqing, Li Huo, Huang Bo, Tian Guangzhao, Liu Shuyun, Sui Xiang, Huang Jingxiang, Guo Quanyi, Lu Xiaobo. Decellularized porcine skin matrix for tissue-engineered meniscus scaffold [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3473-3478. |
| [13] | Mo Jianling, He Shaoru, Feng Bowen, Jian Minqiao, Zhang Xiaohui, Liu Caisheng, Liang Yijing, Liu Yumei, Chen Liang, Zhou Haiyu, Liu Yanhui. Forming prevascularized cell sheets and the expression of angiogenesis-related factors [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3479-3486. |
| [14] | Liu Chang, Li Datong, Liu Yuan, Kong Lingbo, Guo Rui, Yang Lixue, Hao Dingjun, He Baorong. Poor efficacy after vertebral augmentation surgery of acute symptomatic thoracolumbar osteoporotic compression fracture: relationship with bone cement, bone mineral density, and adjacent fractures [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3510-3516. |
| [15] | Liu Liyong, Zhou Lei. Research and development status and development trend of hydrogel in tissue engineering based on patent information [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(22): 3527-3533. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||