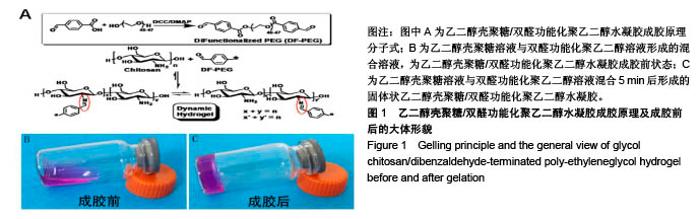
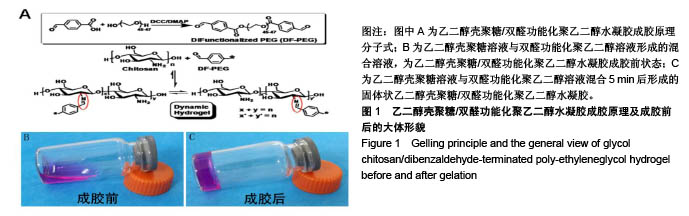
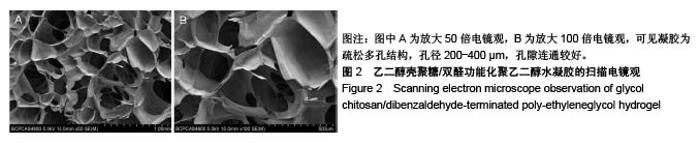
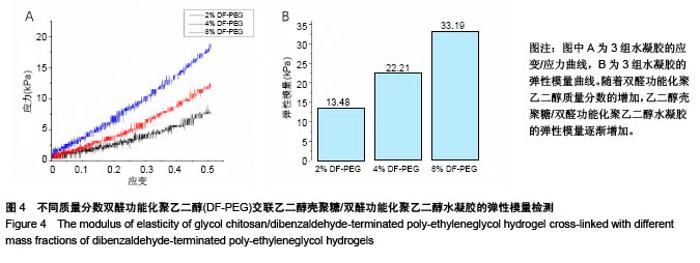
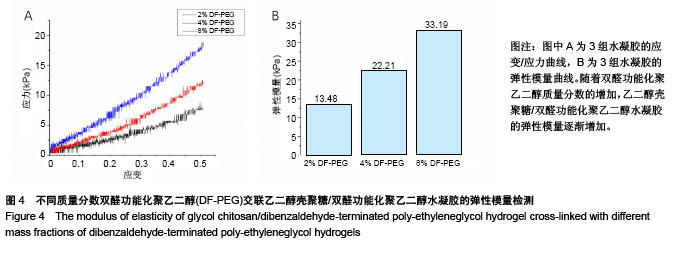
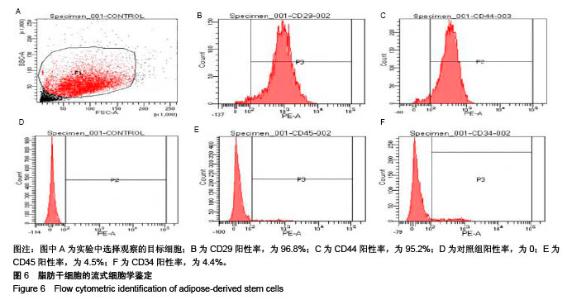
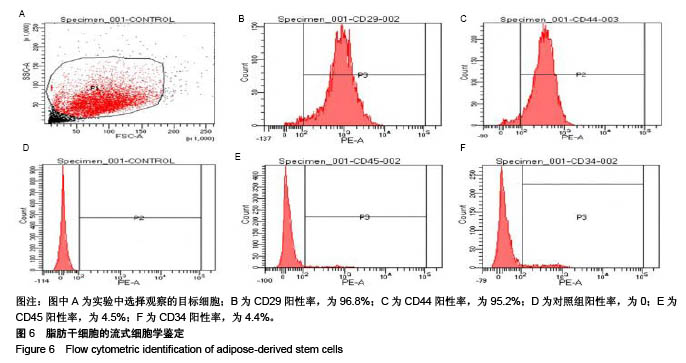
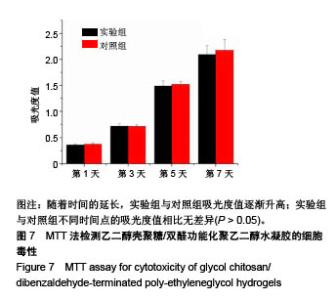
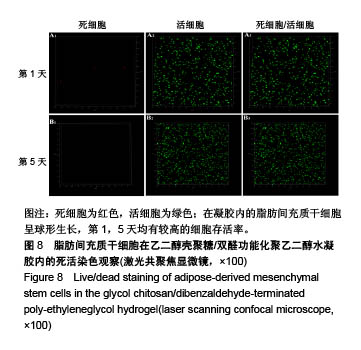
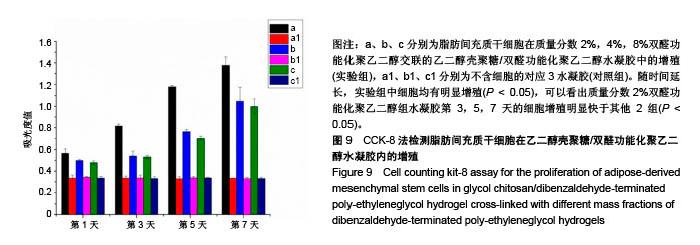
| [1] Rai V, Dilisio MF, Dietz NE, et al. Recent strategies in cartilage repair: A systemic review of the scaffold development and tissue engineering. J Biomed Mater Res A. 2017;105(8):2343-2354. [2] Laudy AB, Bakker EW, Rekers M, et al. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015; 49(10):657-672. [3] Lynch TS, Patel RM, Benedick A, et al. Systematic review of autogenous osteochondral transplant outcomes. Arthroscopy. 2015;31(4):746-754. [4] Oussedik S, Tsitskaris K, Parker D. Treatment of articular cartilage lesions of the knee by microfracture or autologous chondrocyte implantation: a systematic review. Arthroscopy. 2015;31(4):732-744. [5] Johnstone B, Alini M, Cucchiarini M, et al. Tissue engineering for articular cartilage repair--the state of the art. Eur Cell Mater. 2013;25:248-267. [6] 刘雪婷,支晓亮,白春雨,等.脂肪间充质干细胞生物学特性及分泌功能研究[J].农业科学与技术,2016,17(6):1331-1335.[7] Kasir R, Vernekar VN, Laurencin CT. Regenerative Engineering of Cartilage Using Adipose-Derived Stem Cells. Regen Eng Transl Med. 2015;1(1):42-49. [8] Pleumeekers MM, Nimeskern L, Koevoet JLM, et al. Trophic effects of adipose-tissue-derived and bone-marrow-derived mesenchymal stem cells enhance cartilage generation by chondrocytes in co-culture. PloS One. 2018;13(2):1-23. [9] Zhang J, Du C, Guo W, et al. Adipose Tissue-Derived Pericytes for Cartilage Tissue Engineering. Curr Stem Cell Res Ther. 2017;12(6):513-521. [10] Yang J, Zhang YS, Yue K, et al. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater. 2017;57:1-25. [11] Liu M, Zeng X, Ma C, et al. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017;5(2):75-94. [12] Zhang Y, Tao L, Li S, et al. Synthesis of multiresponsive and dynamic chitosan-based hydrogels for controlled release of bioactive molecules. Biomacromolecules. 2011;12(8): 2894-2901. [13] Li Y, Zhang Y, Shi F, et al. Modulus-regulated 3D-cell proliferation in an injectable self-healing hydrogel. Colloids Surf B Biointerfaces. 2017;149:168-173. [14] Qi C, Liu J, Jin Y, et al. Photo-crosslinkable, injectable sericin hydrogel as 3D biomimetic extracellular matrix for minimally invasive repairing cartilage. Biomaterials. 2018;163:89-104. [15] Sun AX, Lin H, Fritch MR, et al. Chondrogenesis of human bone marrow mesenchymal stem cells in 3-dimensional, photocrosslinked hydrogel constructs: Effect of cell seeding density and material stiffness. Acta Biomaterialia. 2017;58: 302-311. |