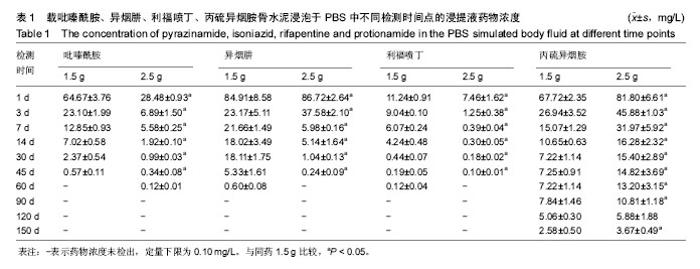
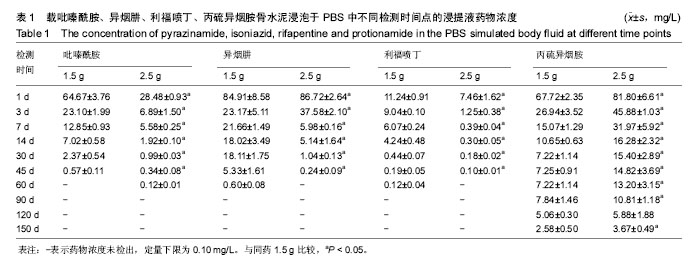
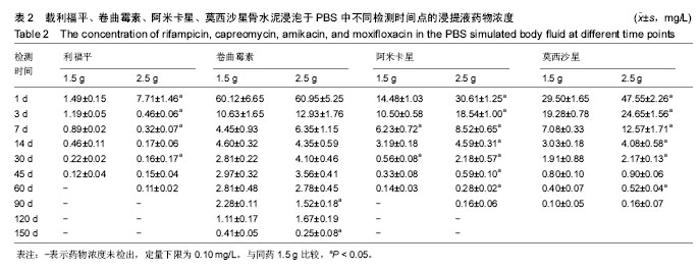
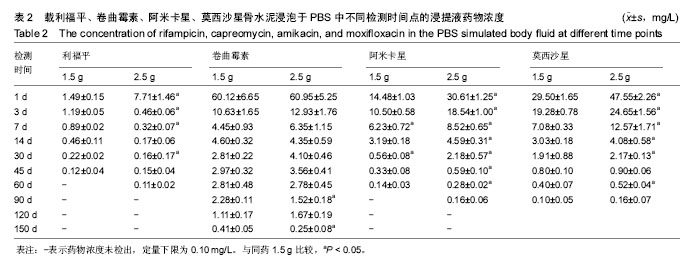
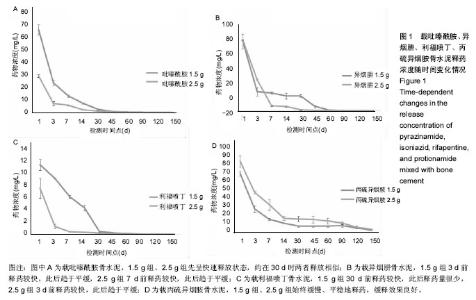
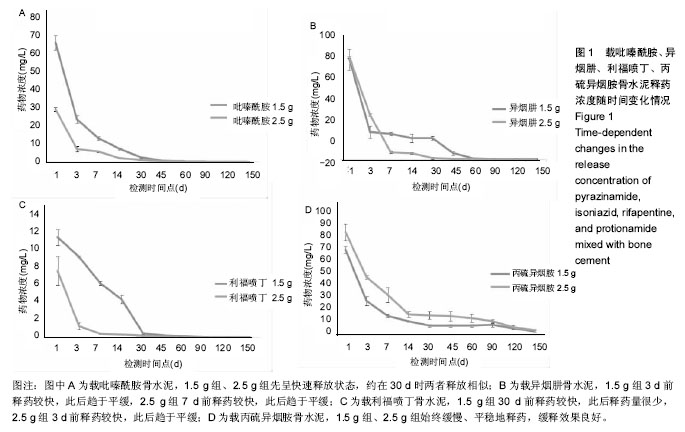
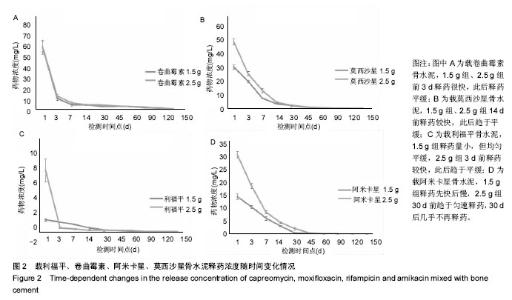
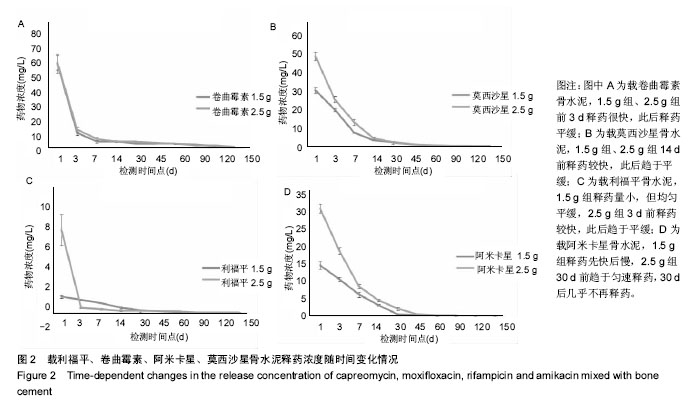
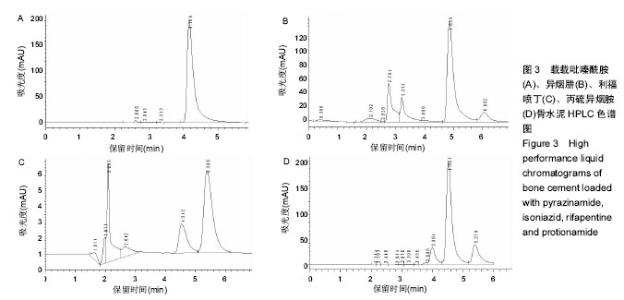
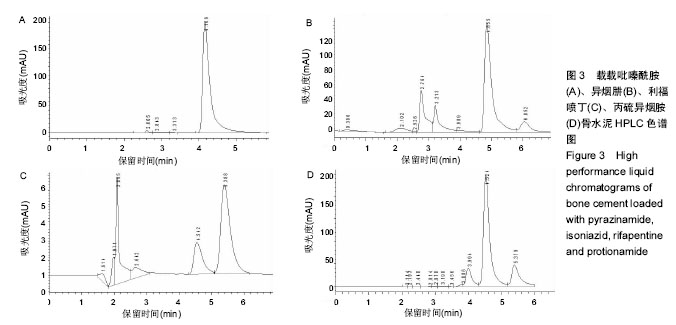
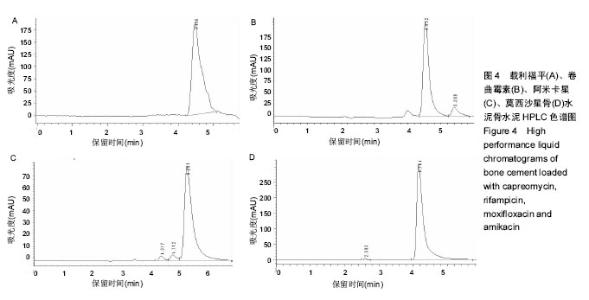
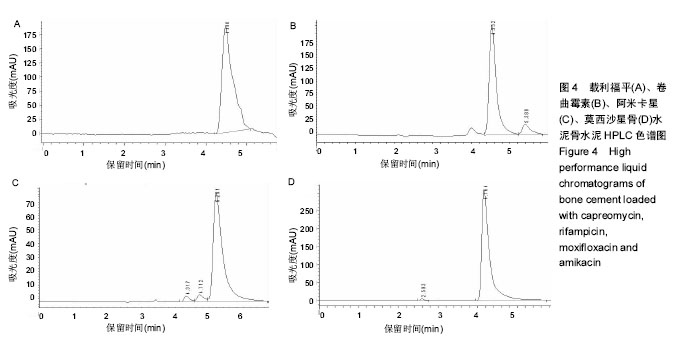
| [1] Zhang YC,Zhang H.One-stage total joint arthropLasty for patients with active tuberculosis.Orthopedics. 2013;36(5):328-330.[2] Hardinge K,WiLLiams D,Etienne A,et al.Conversion of fusion hips to Low friction arthroplasty.Bone Joint Surg. 1977;59(4):385-392.[3] Zeng M,Hu Y,Leng Y,et al.Cementless total hip arthroplasty in advanced tuberculosis of the hip.Int Orthop. 2015;39(11): 2103-2107.[4] 毕红宾,王永清,赵志辉,等.一期病灶清除人工全髋关节置换术治疗活动性髋关节结核[J].中国修复重建外科杂志, 2014,28(8):938-941.[5] 周胜虎,甄平,沈伟伟,等.晚期活动性髋关节结核全髋关节置换术的临床研究[J].中华关节外科杂志:电子版,2016,10(2):1-6.[6] 宋向伟,王骞,王自立,等.脊柱结核彻底病灶清除术后3~4.5个月超短程化疗方案的疗效观察[J].中国脊柱脊髓杂志, 2017,27(4): 326-332.[7] Ge Z,Wang Z,Wei M.Measurement of the concentration of three antituberculosis drugs in thefocus of spinal tuberculosis. Eur Spine J. 2008;17(11):1482-1487.[8] Oga M,Arizono T,Takasita M,et al.Evaluation of the risk of instrumentation as a foreign body in spinal tuberculosis.Clinical and biologic study.Spine(Phila Pa 1976). 1993;18(13):1890-1894.[9] 王骞,刘海涛,王自立,等.聚乳酸/聚乙醇酸共聚物涂饰载三联抗结核药人工骨体外释药对比[J].中国组织工程研究, 2017,21(6):911-916.[10] 王骞,耿广起,丛晓明,等.载三联抗痨药硫酸钙/聚氨基酸缓释材料在兔脊柱结核模型体内的缓释性能[J].中国组织工程研究, 2017,21(10): 1520-1526.[11] 刘海涛,施建党,王骞,等.硫酸钙/聚氨基酸复合三联抗痨药人工缓释材料的制备及物理性能测定[J].中国矫形外科杂志, 2015,23(21): 1984-1988.[12] Vora C,Patadia R,MittaL K,et al.Recent patents and advances on anti-tuberculosis drug deLivery and formulations.Recent Pat Drug Devil Formul.2013;7(2):138-149.[13] BuchhoLz HW,EngeLbrecht H.Depot effects of various antibiotics mixed with PaLacos resins.German Chirurg. 1970;41(11): 511-515.[14] 刘海涛,施建党,王骞,等.载三联抗结核药物硫酸钙/聚氨基酸人工材料体外缓释性能的观察[J].中国脊柱脊髓杂志, 2015,25(3):239-244.[15] 马荣,陈振,戈朝晖,等.载异烟肼、利福平白蛋白纳米粒的制备及其体外释药研究[J].第三军医大学学报, 2013,35(21):2336-2339.[16] 赵晨,王骞,施建党,等.复合三联抗结核药涂层材料(HRZ/PLGA)在大鼠体内释药特性观察[J].中国脊柱脊髓杂志, 2016,26(7):635-641.[17] 朱荣,龚俊.HPLC测定盐酸莫西沙星的含量和有关物质[J].华西药学杂志, 2017,32(4):398-400.[18] 王骞,马文鑫.骨关节结核:骨病灶药物分布特点及缓释材料[J].中国组织工程研究,2015,19(48):7859-7859.[19] 李国华,丁黎明,张斌,等.含抗生素可活动关节骨水泥对全膝关节置换过程中血沉、C反应蛋白及关节功能的影响[J].中国组织工程研究, 2016,20(3):319-323.[20] 马文鑫,金卫东,王骞,等.载利福平、异烟肼、吡嗪酰胺、莫西沙星骨水泥物理性能及洗脱性能的体外研究[J].中华骨科杂志, 2016,36(11): 735-744.[21] 李涛,翁习生.抗生素骨水泥在人工关节置换术后感染中应用研究的系统性综述[J].中国矫形外科杂志, 2014,22(20):1868-1874.[22] 何亮,党晓谦.木糖醇对庆大霉素骨水泥释放及生物力学的影响研究[J].中国现代医学杂志,2014,24(32):9-14.[23] 虢剑,王骞,马文鑫,等.32例脊柱内固定术后急性感染的临床分析[J].宁夏医科大学学报,2016,38(12):1458-1461.[24] 马文鑫,王骞,王自立,等.脊柱内固定术后感染的治疗[J].中国矫形外科杂志,2016,24(15):1357-1362.[25] 施建党,刘园园,王骞,等.病椎固定治疗胸、腰椎结核的疗效分析[J].中华骨科杂志,2016,36(11):681-690.[26] Jin W,Wang Q,Wang Z,et al.Complete debridement for treatment of thoracolumbar spinal tuberculosis: a clinical curative effect observation.Spine J.2014;14(6):964-970. [27] 闫军法,王骞,王自立,等.涂饰PLGA载三联抗痨药人工骨的体外释药研究[J].宁夏医科大学学报,2015,37(6):661-664.[28] 何胤,杨宗强,王骞,等.复合三联抗结核药聚乳酸-羟基乙酸缓释微球的制备及体外释药特性[J].中国脊柱脊髓杂志, 2015,25(5):456-461. |