Chinese Journal of Tissue Engineering Research ›› 2018, Vol. 22 ›› Issue (17): 2733-2739.doi: 10.3969/j.issn.2095-4344.0507
Previous Articles Next Articles
Core decompression combined with autologous bone marrow mesenchymal stem cells for femoral head necrosis: a meta-analysis of safety and efficacy
Wang Qian1, 2, Huang Guo-xin1, Chen Lei1, Li De-sheng2, Ai Jin-wei1, 2, 3, Pei Bin1, 2, 3
- 1Evidence-Based Medicine Center, 2Department of Plastic Surgery, 3Research Office of Plastic Surgery and Orthopedics, Xiangyang No.1 People’s Hospital, Hubei University of Medicine, Xiangyang 441000, Hubei Province, China
-
Revised:2018-01-26Online:2018-06-18Published:2018-06-18 -
Contact:Pei Bin, Master, Master’s supervisor, Chief physician, Professor, Evidence-Based Medicine Center, Xiangyang No.1 People’s Hospital, Hubei University of Medicine, Xiangyang 441000, Hubei Province, China -
About author:Wang Qian, Master, Attending physician, Evidence-Based Medicine Center, Xiangyang No.1 People’s Hospital, Hubei University of Medicine, Xiangyang 441000, Hubei Province, China. Huang Guo-xin, Evidence-Based Medicine Center, Xiangyang No.1 People’s Hospital, Hubei University of Medicine, Xiangyang 441000, Hubei Province, China. Wang Qian and Huang Guo-xin contributed equally to this work. -
Supported by:the General Program of Western Medicine of the Health and Family Planning Committee of Hubei Province in 2015-2016, No. WJ2015MB187; the Key Research and Teaching Program of Hubei University of Medicine, No. 2015025; the Scientific Research Plan of Xiangyang City, No. 2010GG3A21
CLC Number:
Cite this article
Wang Qian, Huang Guo-xin, Chen Lei, Li De-sheng, Ai Jin-wei, Pei Bin. Core decompression combined with autologous bone marrow mesenchymal stem cells for femoral head necrosis: a meta-analysis of safety and efficacy[J]. Chinese Journal of Tissue Engineering Research, 2018, 22(17): 2733-2739.
share this article
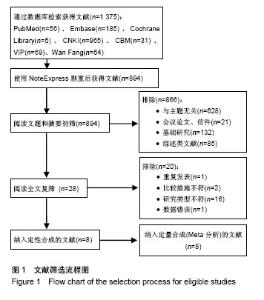
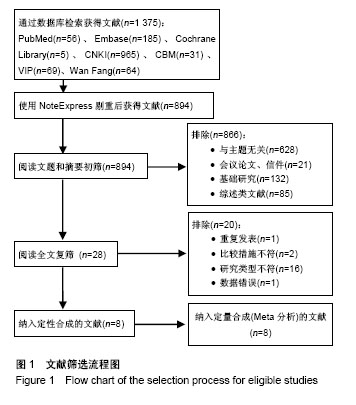
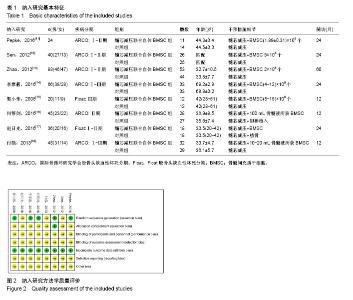
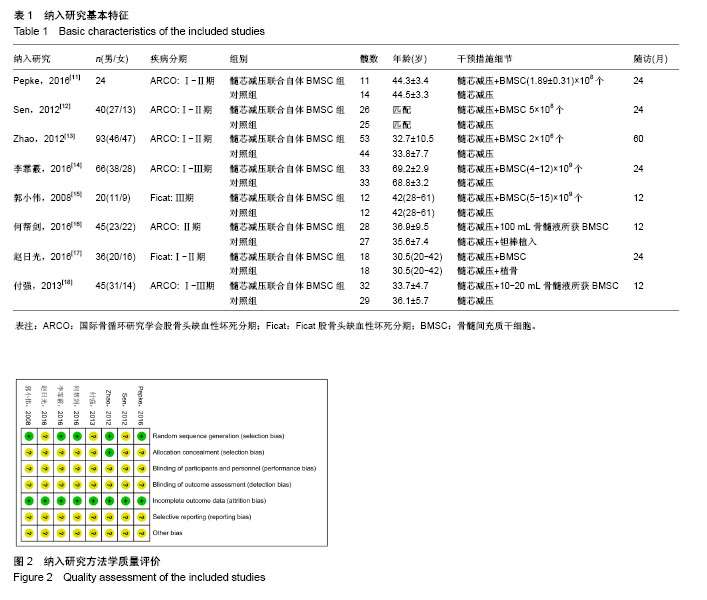
2.1 文献检索结果 初检出文献2 355篇,排除重复文献后剩余894篇,经逐层筛选,最终纳入8个临床随机对照试验[11-18]。其中,英文文献3篇[11-13],中文献5篇[14-18]。文献筛选流程图见图1。 2.2 纳入研究基本特征 8个临床随机对照试验共包含股骨头缺血坏死患者323例395髋,股骨头坏死ARCO分期为Ⅰ-Ⅲ期,其中髓芯减压联合自体骨髓间充质干细胞组193髋,髓芯减压组202髋。骨髓间充质干细胞均于髓芯减压同时注入股骨头缺血坏死区,给予细胞数量级最低为106,最多为109,3个研究仅描述抽取骨髓的毫升数[16-18],未描述具体骨髓间充质干细胞数目。随访时间最短为12个月[15-16,18],最长为60个月[13]。纳入研究基本特征见表1。按Cochrane系统评价员手册5.1.0版随机对照试验偏倚风险评估工具,纳入研究总体为中等质量研究,纳入研究方法学质量评价见图2。"
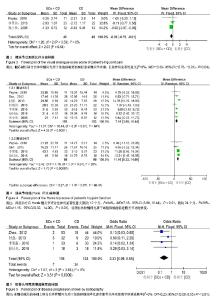
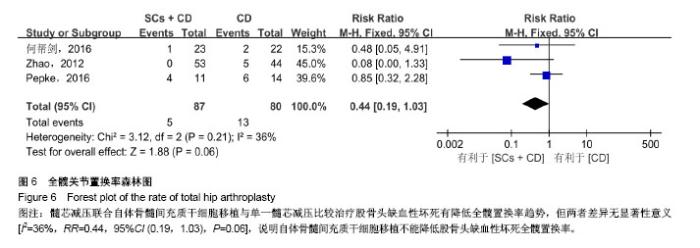
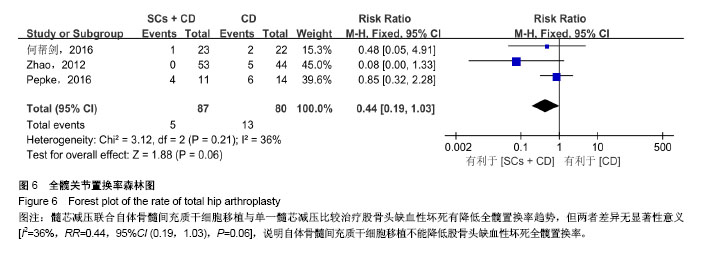
2.3 Meta分析结果 2.3.1 髋关节疼痛 3个研究运用目测类比评分评价了髓芯减压联合自体骨髓间充质干细胞移植与单一髓芯减压对股骨头缺血性坏死髋关节疼痛的影响[11,15-16]。Meta分析结果显示髓芯减压联合自体骨髓间充质干细胞移植更能减轻患者髋关节疼痛,且差异有显著性意义[I2=0%,MD=-0.39,95%CI (-0.76,-0.01),P=0.04],见图3。因纳入研究数量较少,未按随访时间进行亚组分析。 2.3.2 髋关节功能评分 7个研究运用Harris髋关节评分比较了两组治疗后髋关节功能差异[11-12,14-18]。其中,7个研究报道了随访12个月时Harris髋关节评分[11-12,14-18],4个研究报道了随访24个月时Harris髋关节评分[11-12,14,17]。Meta分析结果显示:两组治疗后Harris髋关节评分差异有显著性意义[随访12个月:I2=64%,MD=7.16,95%CI (3.88,10.44),P < 0.01;随访24个月:I2=28%,MD=11.16,95%CI (8.32,14.00),P < 0.01],见图4;随访12个月时各研究间异质性较大,分析1个研究对异质性影响较明显[18],给予排除进行敏感性分析,结果性质未发生明显变化[I2=0%,MD=6.00,95%CI (4.05,7.96),P < 0.01]。说明自体骨髓间充质干细胞移植能促进髋关节功能恢复。 2.3.3 病情进展率 4个研究运用影像学检查根据ARCO/ Ficat分期评价两组患者股骨头坏死病情进展率[13-14,17-18]。Meta分析结果显示,在髓芯减压的基础上联合自体骨髓间充质干细胞移植能降低患者股骨头坏死影像学病情进展率 [I2=0%,OR=0.23,95%CI (0.09,0.55),P < 0.01],见图5。 2.3.4 全髋关节置换率 3个研究报道了随访过程中全髋关节置换人数[11,13,16]。Meta分析结果显示髓芯减压联合自体骨髓间充质干细胞移植与单一髓芯减压比较治疗股骨头缺血性坏死有降低全髋置换率趋势,但两者差异无显著性意义[I2=36%,RR=0.44,95%CI (0.19,1.03),P=0.06],见图6。说明自体骨髓间充质干细胞移植不能降低股骨头缺血性坏死全髋置换率。 2.3.5 并发症率 4个研究报道了治疗过程中并发症发生情况[11-13,15],均显示自体骨髓间充质干细胞移植过程中及随访期间无明显并发症发生,说明对股骨头缺血性坏死患者在髓芯减压基础上运用自体骨髓间充质干细胞移植是安全的。"
| [1] Ando W, Yamamoto K, Koyama T, et al. Radiologic and Clinical Features of Misdiagnosed Idiopathic Osteonecrosis of the Femoral Head. Orthopedics. 2017;40(1):e117-e123.[2] Kubo T, Ueshima K, Saito M, et al. Clinical and basic research on steroid-induced osteonecrosis of the femoral head in Japan. J Orthop Sci. 2016;21(4):407-413.[3] Tripathy SK, Goyal T, Sen RK. Management of femoral head osteonecrosis: Current concepts. Indian J Orthop. 2015;49(1): 28-45.[4] Silva LL, Castelar M, Matos MA. Quality of Life in Pediatric Patients with Avascular Necrosis of the Femoral Head. Ortop Traumatol Rehabil. 2016;18(5):445-449.[5] Roth A, Beckmann J, Bohndorf K, et al. S3-Guideline non-traumatic adult femoral head necrosis. Arch Orthop Trauma Surg. 2016;136(2):165-174.[6] Joint Surgery Group of the Orthopaedic Branch of the Chinese Medical Association. Guideline for Diagnostic and Treatment of Osteonecrosis of the Femoral Head. Orthop Surg. 2015;7(3):200-207.[7] Hong YC, Zhong HM, Lin T, et al. Comparison of core decompression and conservative treatment for avascular necrosis of femoral head at early stage: a meta-analysis. Int J Clin Exp Med. 2015;8(4):5207-5216.[8] Suh KT, Kim SW, Roh HL, et al. Decreased osteogenic differentiation of mesenchymal stem cells in alcohol-induced osteonecrosis. Clin Orthop Relat Res. 2005;(431):220-225.[9] Hernigou P, Beaujean F. Abnormalities in the bone marrow of the iliac crest in patients who have osteonecrosis secondary to corticosteroid therapy or alcohol abuse. J Bone Joint Surg Am. 1997;79(7):1047-1053.[10] Zeng X, Zhang Y, Kwong JS, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015; 8(1):2-10.[11] Pepke W, Kasten P, Beckmann NA, et al. Core Decompression and Autologous Bone Marrow Concentrate for Treatment of Femoral Head Osteonecrosis: A Randomized Prospective Study. Orthop Rev (Pavia). 2016; 8(1):6162.[12] Sen RK, Tripathy SK, Aggarwal S, et al. Early results of core decompression and autologous bone marrow mononuclear cells instillation in femoral head osteonecrosis: a randomized control study. J Arthroplasty. 2012;27(5):679-686.[13] Zhao D, Cui D, Wang B, et al. Treatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-derived and cultured mesenchymal stem cells. Bone. 2012;50(1):325-330.[14] 李霏霰,吴齐英,李新志,等. 髓芯减压术联合自体骨髓干细胞移植治疗老年缺血性股骨头坏死的疗效[J]. 中国老年学杂志, 2016,36(22):5671-5672.[15] 郭小伟,吴卫新,洪云飞,等. 髓芯减压并自体骨髓干细胞移植治疗股骨头缺血性坏死[J]. 医药论坛杂志,2008,29(5):19-21.[16] 何帮剑,厉驹,吕一,等. 髓芯减压联合干细胞移植与钽棒植入术治疗Ⅱ期非创伤性股骨头坏死的临床研究[J]. 中国骨伤, 2016,29(12):1119-1124.[17] 赵日光,刘宏滨,韩冰,等. 髓芯减压联合自体骨髓干细胞移植治疗早期股骨头缺血性坏死的疗效观察[J]. 人民军医, 2016, 59(4):372-373.[18] 付强,闫世杰,王江泳,等. 髓芯减压联合自体骨髓间充质干细胞治疗45例股骨头无菌性坏死的临床疗效分析[J]. 现代生物医学进展,2013,13(25):4925-4928.[19] Hu LB, Huang ZG, Wei HY, et al. Osteonecrosis of the femoral head: using CT, MRI and gross specimen to characterize the location, shape and size of the lesion. Br J Radiol. 2015;88(1046):20140508.[20] Kubo Y, Yamamoto T, Motomura G, et al. Patient-reported outcomes of femoral osteotomy and total hip arthroplasty for osteonecrosis of the femoral head: a prospective case series study. Springerplus. 2016;5(1):1880.[21] Sadile F, Bernasconi A, Russo S, et al. Core decompression versus other joint preserving treatments for osteonecrosis of the femoral head: a meta-analysis. Br Med Bull. 2016;118(1):33-49.[22] Gillespy T 3rd, Genant HK, Helms CA. Magnetic resonance imaging of osteonecrosis. Radiol Clin North Am. 1986;24(2): 193-208.[23] Ganczarczyk ML, Lee P, Fornasier VL. Early diagnosis of osteonecrosis in systemic lupus erythematosus with magnetic resonance imaging. Failure of core decompression. J Rheumatol. 1986;13(4):814-817.[24] Hungerford DS. Core decompression of the femoral head for osteonecrosis. J Bone Joint Surg Am. 1988;70(3): 474-475.[25] Steinberg ME, Larcom PG, Strafford B, et al. Core decompression with bone grafting for osteonecrosis of the femoral head. Clin Orthop Relat Res. 2001;(386):71-78.[26] Arbeloa-Gutierrez L, Dean CS, Chahla J, et al. Core Decompression Augmented With Autologous Bone Marrow Aspiration Concentrate for Early Avascular Necrosis of the Femoral Head. Arthrosc Tech. 2016;5(3):e615-620.[27] Kinnaird T, Stabile E, Burnett MS, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94(5):678-685.[28] Lebouvier A, Poignard A, Cavet M, et al. Development of a simple procedure for the treatment of femoral head osteonecrosis with intra-osseous injection of bone marrow mesenchymal stromal cells: study of their biodistribution in the early time points after injection. Stem Cell Res Ther. 2015;6:68.[29] 李子荣. 股骨头坏死的ARCO分期[J]. 中华外科杂志,1996, 34(3):186-187. |
| [1] | Hu Kai, Qiao Xiaohong, Zhang Yonghong, Wang Dong, Qin Sihe. Treatment of displaced intra-articular calcaneal fractures with cannulated screws and plates: a meta-analysis of 15 randomized controlled trials [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1465-1470. |
| [2] | Huang Dengcheng, Wang Zhike, Cao Xuewei. Comparison of the short-term efficacy of extracorporeal shock wave therapy for middle-aged and elderly knee osteoarthritis: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1471-1476. |
| [3] | Zhang Tongtong, Wang Zhonghua, Wen Jie, Song Yuxin, Liu Lin. Application of three-dimensional printing model in surgical resection and reconstruction of cervical tumor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1335-1339. |
| [4] | Chen Junming, Yue Chen, He Peilin, Zhang Juntao, Sun Moyuan, Liu Youwen. Hip arthroplasty versus proximal femoral nail antirotation for intertrochanteric fractures in older adults: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1452-1457. |
| [5] | Chen Jinping, Li Kui, Chen Qian, Guo Haoran, Zhang Yingbo, Wei Peng. Meta-analysis of the efficacy and safety of tranexamic acid in open spinal surgery [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(9): 1458-1464. |
| [6] | Wang Yongsheng, Wu Yang, Li Yanchun. Effect of acute high-intensity exercise on appetite hormones in adults: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(8): 1305-1312. |
| [7] | Hou Jingying, Yu Menglei, Guo Tianzhu, Long Huibao, Wu Hao. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival and vascularization through the activation of HIF-1α/MALAT1/VEGFA pathway [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 985-990. |
| [8] | Liang Xueqi, Guo Lijiao, Chen Hejie, Wu Jie, Sun Yaqi, Xing Zhikun, Zou Hailiang, Chen Xueling, Wu Xiangwei. Alveolar echinococcosis protoscolices inhibits the differentiation of bone marrow mesenchymal stem cells into fibroblasts [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 996-1001. |
| [9] | Geng Yao, Yin Zhiliang, Li Xingping, Xiao Dongqin, Hou Weiguang. Role of hsa-miRNA-223-3p in regulating osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1008-1013. |
| [10] | Lun Zhigang, Jin Jing, Wang Tianyan, Li Aimin. Effect of peroxiredoxin 6 on proliferation and differentiation of bone marrow mesenchymal stem cells into neural lineage in vitro [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1014-1018. |
| [11] | Zhu Xuefen, Huang Cheng, Ding Jian, Dai Yongping, Liu Yuanbing, Le Lixiang, Wang Liangliang, Yang Jiandong. Mechanism of bone marrow mesenchymal stem cells differentiation into functional neurons induced by glial cell line derived neurotrophic factor [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1019-1025. |
| [12] | Pei Lili, Sun Guicai, Wang Di. Salvianolic acid B inhibits oxidative damage of bone marrow mesenchymal stem cells and promotes differentiation into cardiomyocytes [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1032-1036. |
| [13] | Wang Shiqi, Zhang Jinsheng. Effects of Chinese medicine on proliferation, differentiation and aging of bone marrow mesenchymal stem cells regulating ischemia-hypoxia microenvironment [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1129-1134. |
| [14] | Zeng Yanhua, Hao Yanlei. In vitro culture and purification of Schwann cells: a systematic review [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1135-1141. |
| [15] | Kong Desheng, He Jingjing, Feng Baofeng, Guo Ruiyun, Asiamah Ernest Amponsah, Lü Fei, Zhang Shuhan, Zhang Xiaolin, Ma Jun, Cui Huixian. Efficacy of mesenchymal stem cells in the spinal cord injury of large animal models: a meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(7): 1142-1148. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||