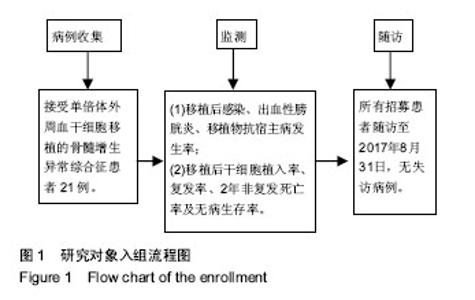
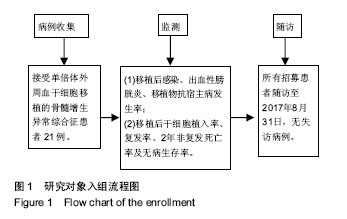
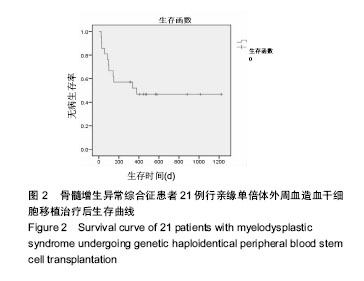
| [1] Garcia-Manero G, Shan J, Faderl S, et al. A prognostic score for patients with lower risk myelodysplastic syndrome. Leukemia. 2008;22(3):538-543.[2] Malcovati L, Hellström-Lindberg E, Bowen D, et al. Diagnosis and treatment of primary myelodysplastic syndromes in adults: recommendations from the European LeukemiaNet. Blood. 2013;122(17):2943-2964.[3] Greenberg PL, Attar E, Bennett JM, et al. NCCN Clinical Practice Guidelines in Oncology: myelodysplastic syndromes. J Natl Compr Canc Netw. 2011;9(1):30-56.[4] de Witte T, Hagemeijer A, Suciu S, et al. Value of allogeneic versus autologous stem cell transplantation and chemotherapy in patients with myelodysplastic syndromes and secondary acute myeloid leukemia. Final results of a prospective randomized European Intergroup Trial. Haematologica. 2010;95(10):1754-1761.[5] Lim Z, Brand R, Martino R, et al. Allogeneic hematopoietic stem-cell transplantation for patients 50 years or older with myelodysplastic syndromes or secondary acute myeloid leukemia. J Clin Oncol. 2010;28(3):405-411.[6] Champlin RE, Schmitz N, Horowitz MM, et al. Blood stem cells compared with bone marrow as a source of hematopoietic cells for allogeneic transplantation. IBMTR Histocompatibility and Stem Cell Sources Working Committee and the European Group for Blood and Marrow Transplantation (EBMT). Blood. 2000;95(12):3702-3709.[7] Lazarus HM, Advani AS. When, how, and what cell source for hematopoietic cell transplantation in first complete remission adult acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2012;2012:382-388.[8] Gyurkocza B, Deeg HJ. Allogeneic hematopoietic cell transplantation for MDS: for whom, when and how. Blood Rev. 2012;26(6):247-254.[9] 王莹,樊星,王苓,等. 异基因造血干细胞移植后死亡原因分析[J]. 内科理论与实践, 2014,9(4): 270-273.[10] 宋阿霞,杨栋林,魏嘉璘,等. 异基因造血干细胞移植治疗75例完全缓解期急性髓系白血病的疗效及预后分析[J]. 中国实验血液学杂志, 2010,18(1): 161-166.[11] Gratwohl A, Brand R, Frassoni F, et al. Cause of death after allogeneic haematopoietic stem cell transplantation (HSCT) in early leukaemias: an EBMT analysis of lethal infectious complications and changes over calendar time. Bone Marrow Transplant. 2005;36(9):757-769.[12] Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20): 2391-2405.[13] Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120(12):2454-2465.[14] Sung AD, Chao NJ. Concise review: acute graft-versus-host disease: immunobiology, prevention, and treatment. Stem Cells Transl Med. 2013;2(1):25-32.[15] Carpenter PA, Kitko CL, Elad S, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: V. The 2014 Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant. 2015;21(7):1167-1187.[16] 桑丽娜,孙玲,李英梅,等. 造血干细胞移植术后出血性膀胱炎的临床观察[J]. 中华血液学杂志, 2014, 35(8): 747-749.[17] 何海燕. 父母供者外周血单倍体干细胞移植治疗儿童复发难治急性白血病[D].郑州:郑州大学,2015.[18] 万鼎铭,周雪芳,谢新生,等. 异基因造血干细胞移植后肠球菌相关性腹泻的临床分析[J]. 郑州大学学报:医学版, 2014,49(2): 284-287.[19] Bedi A, Miller CB, Hanson JL, et al. Association of BK virus with failure of prophylaxis against hemorrhagic cystitis following bone marrow transplantation. J Clin Oncol. 1995; 13(5):1103-1109.[20] Passweg JR, Baldomero H, Bader P, et al. Hematopoietic stem cell transplantation in Europe 2014: more than 40 000 transplants annually. Bone Marrow Transplant. 2016;51(6): 786-792.[21] Della Porta MG, Alessandrino EP, Bacigalupo A, et al. Predictive factors for the outcome of allogeneic transplantation in patients with MDS stratified according to the revised IPSS-R. Blood. 2014;123(15):2333-2342.[22] 王昱,黄晓军. 单倍型造血干细胞移植治疗骨髓增生异常综合征的进展[J]. 中华血液学杂志, 2017, 38(4): 348-351.[23] Wang Y, Wang HX, Lai YR, et al. Haploidentical transplant for myelodysplastic syndrome: registry-based comparison with identical sibling transplant. Leukemia. 2016;30(10): 2055-2063.[24] Di Stasi A, Milton DR, Poon LM, et al. Similar transplantation outcomes for acute myeloid leukemia and myelodysplastic syndrome patients with haploidentical versus 10/10 human leukocyte antigen-matched unrelated and related donors. Biol Blood Marrow Transplant. 2014;20(12):1975-1981.[25] 卢岳,吴彤,赵艳丽. 不同异基因造血干细胞移植方式治疗167例中高危骨髓增生异常综合征疗效比较[J]. 中华血液学杂志, 2017, 38(4): 301-306.[26] Guardiola P, Runde V, Bacigalupo A, et al. Retrospective comparison of bone marrow and granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells for allogeneic stem cell transplantation using HLA identical sibling donors in myelodysplastic syndromes. Blood. 2002;99(12):4370-4378.[27] Pidala J, Anasetti C, Kharfan-Dabaja MA, et al. Decision analysis of peripheral blood versus bone marrow hematopoietic stem cells for allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15(11): 1415-1421.[28] Nevill TJ, Shepherd JD, Sutherland HJ, et al. IPSS poor-risk karyotype as a predictor of outcome for patients with myelodysplastic syndrome following myeloablative stem cell transplantation. Biol Blood Marrow Transplant. 2009;15(2): 205-213.[29] 沈耀耀,刘跃均,吴德沛,等. 异基因造血干细胞移植后免疫重建的临床研究及急性移植物抗宿主病的危险因素分析[J]. 白血病•淋巴瘤, 2015, 24(6): 346-351.[30] Handgretinger R. Haploidentical transplantation: the search for the best donor. Blood. 2014;124(6):827-828.[31] Platzbecker U. Who benefits from allogeneic transplantation for myelodysplastic syndromes?: new insights. Hematology Am Soc Hematol Educ Program. 2013;2013:522-528. |