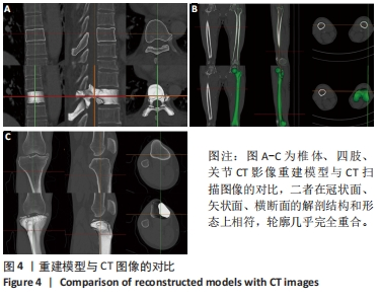
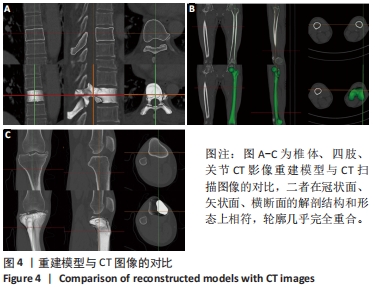
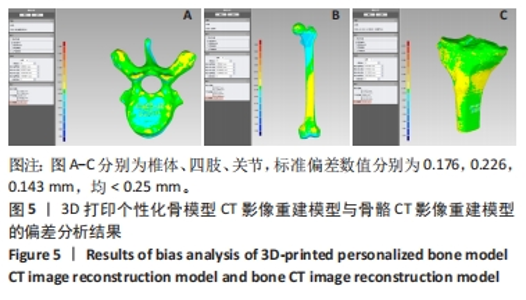
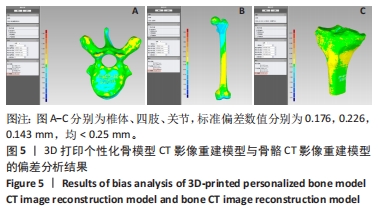
[1] 闵玥, 张家振, 刘斌, 等. 无源植入性骨、关节及口腔硬组织个性化增材制造医疗器械注册技术审查指导原则探讨[J]. 中国医疗器械杂志,2021,45(2): 200-204.
[2] JIN ZBY, HE CF, FU JZ, et al. Balancing the customization and standardization: exploration and layout surrounding the regulation of the growing field of 3D-printed medical devices in China. Biodes Manuf. 2022;5(3):580-606.
[3] 邹运, 韩青, 徐晓麟, 等. 骨科和口腔颌面外科3D打印模型的精度验证和可靠性分析[J]. 吉林大学学报(医学版),2017,43(5):996-1001+1074.
[4] 朱栋梁, 卢建华, 李雪丽, 等. 光固化立体成型法3D打印人体骨骼模型的精度研究[J]. 影像研究与医学应用,2019,3(17):244-246.
[5] 李晶, 韩高峰, 王硕, 等. 用于简单病例无托槽隐形矫治器制作的3D打印模型精度研究[J]. 中华口腔正畸学杂志,2021,28(4):184-187.
[6] BROWN GB, CURRIER GF, KADIOGLU O, et al. Accuracy of 3-dimensional printed dental models reconstructed from digital intraoral impressions. Am J Orthod Dentofacial Orthop. 2018;154(5):733-739.
[7] GIUDICE LA, RONSIVALLE V, RUSTICO L, et al. Evaluation of the accuracy of orthodontic models prototyped with entry-level LCD-based 3D printers: a study using surface-based superimposition and deviation analysis. Clin Oral Investig. 2021; 26(1):303-312.
[8] ZHANG ZC, LI PL, CHU FT, et al. Influence of the three-dimensional printing technique and printing layer thickness on model accuracy. J Orofac Orthop. 2019;80(4):194-204.
[9] 国家药品监督管理局. 国家药监局关于发布一次性使用乳腺定位丝注册技术审查等6项注册技术审查指导原则的通告(2020年第48号)[EB/OL]. (2020-07-09)[2023-06-13]. https://www.nmpa.gov.cn/ylqx/ylqxggtg/ylqxzhdyz/20200709154801923.html.
[10] 陈硕平, 易和平, 罗志虹, 等. 高分子3D打印材料和打印工艺[J]. 材料导报, 2016,30(7):54-59.
[11] 张国栋, 杨纪元, 冯新德, 等. 聚乳酸的研究进展[J]. 化学进展,2000,12(1): 89-102.
[12] 唐通鸣, 张政, 邓佳文, 等. 基于FDM的3D打印技术研究现状与发展趋势[J]. 化工新型材料,2015,43(6):228-230+234.
[13] LEDINGHAM AD, ENGLISH JD, AKYALCIN S, et al. Accuracy and mechanical properties of orthodontic models printed 3-dimensionally from calcium sulfate before and after various postprinting treatments. Am J Orthod Dentofacial Orthop. 2016;150(6):1056-1062.
[14] 中华医学会医学工程学分会数字骨科学组. 3D打印骨科模型技术标准专家共识[J]. 中华创伤骨科杂志,2017,19(1):61-64.
[15] 王燎, 戴尅戎. 骨科个体化治疗与3D打印技术[J]. 医用生物力学,2014,29(3): 193-199.
[16] 裴国献. 着力打造3D打印在骨科应用的技术平台[J]. 中华创伤骨科杂志, 2016,18(1):4-5.
[17] GROTH C, KRAVITZ ND, JONES PE, et al. Three-dimensional printing technology. J Clin Orthod. 2014;48(8):475-485.
[18] 韩倩倩, 黄东臣, 杨静, 等. 定制式和患者匹配增材制造医疗器械医工交互全过程构成要素与控制方法研究[J]. 中国药事,2019,33(12):1438-1443.
[19] 郭晓磊, 刘斌. 定制式骨植入物及工具设计开发中的医工交互质量控制要点[J]. 中国医药导报,2018,15(30):160-163+177.
[20] 闵玥. 明确设计要求,指导质量管理[N]. 中国医药报, 2021-07-08(004).
[21] 国家药品监督管理局. 国家药监局 国家卫生健康委关于发布定制式医疗器械监督管理规定(试行)的公告(2019年 第53号)[EB/OL]. (2019-07-04)[2023-06-13]. https://www.nmpa.gov.cn/xxgk/fgwj/xzhgfxwj/ 20190704160701585.html.
[22] 张春光, 么远, 陈晖. 数字化补件在单侧颌面部骨缺损修复中的精确度研究[J]. 现代口腔医学杂志,2021,35(1):26-29.
[23] SHIN SH, KWON JS, SHIM JS, et al. Evaluating the Three-Dimensional Printing Accuracy of Partial-Arch Models According to Outer Wall Thickness: An In Vitro Study. Materials. 2021;14(22):6734.
[24] KENNING KB, RISINGER DC, ENGLISH JD, et al. Evaluation of the dimensional accuracy of thermoformed appliances taken from 3D printed models with varied shell thicknesses: An in vitro study. Int Orthod. 2021;19(1):137-146.
[25] LUENAM S, BANTUCHAI T, KOSIYATRAKUL A, et al. Precision of computed tomography and cartilage-reproducing image reconstruction method in generating digital model for potential use in 3D printing of patient-specific radial head prosthesis: a human cadaver study. 3D Print Med. 2021;7(1):3.
[26] 李丹, 杨胜涛, 袁泉, 等. 3种工艺制作全口义齿基托精确度与固位力的比较[J]. 中华口腔医学杂志,2022,57(9):927-931.
[27] LOFLIN WA, ENGLISH JD, BORDERS C, et al. Effect of print layer height on the assessment of 3D-printed models. Am J Orthod Dentofacial Orthop. 2019;156(2): 283-289.
[28] 国家药品监督管理局. 国家药监局关于发布无源植入性骨、关节及口腔硬组织个性化增材制造医疗器械注册技术审查指导原则的通告(2019年第70号)[EB/OL]. (2019-10-15)[2023-06-13]. https://www.nmpa.gov.cn/ylqx/ylqxggtg/ylqxqtgg/20191015164601944.html.
|